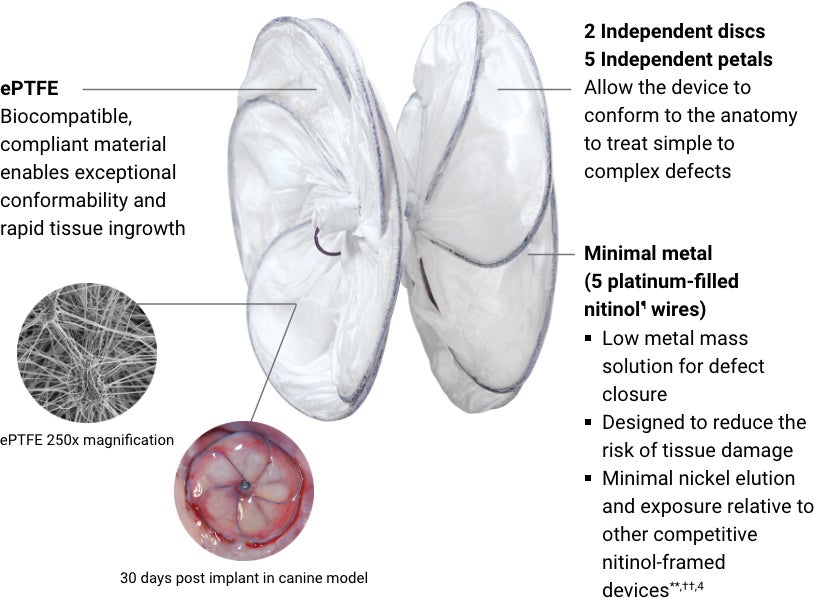
GORE® CARDIOFORM Septal Occluder
The advanced conformable solution for patent foramen ovale (PFO) and ostium secundum atrial septal defect (ASD) closure.

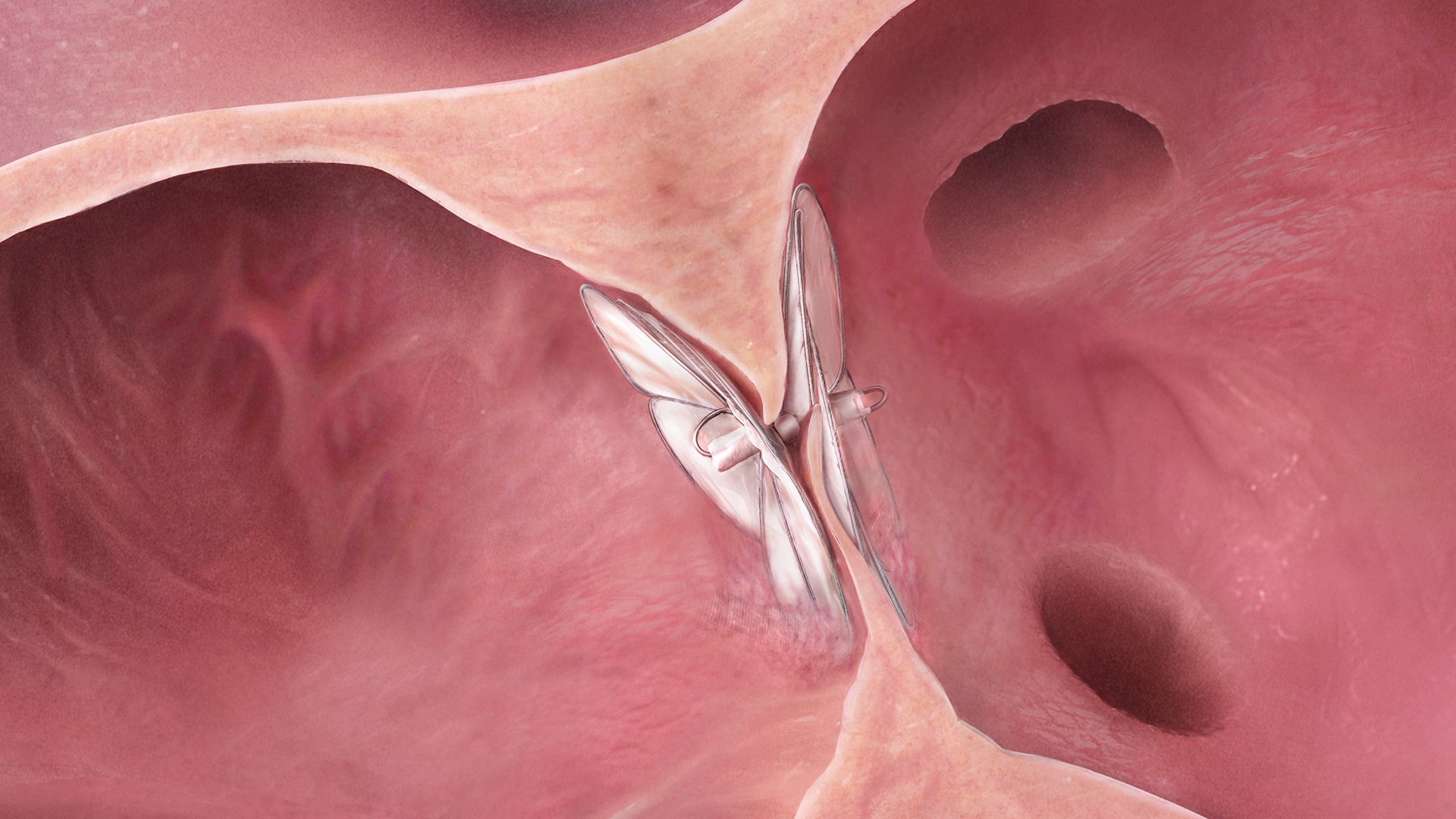
Designed to conform. So the heart doesn’t have to.
The GORE® CARDIOFORM Septal Occluder advances PFO closure with a solution designed to naturally conform to a patient’s unique PFO anatomy — delivering on long-term safety and performance.*,1
The GORE® CARDIOFORM Septal Occluder is a permanently implanted device indicated for the percutaneous, transcatheter closure of atrial septal defects (ASDs), such as ostium secundum and patent foramen ovale.
Long-term safety and performance
0
reported cardiac erosions‡‡
99%
effective closure across anatomiesǂ,§
69%
relative reduction in recurrent stroke
versus medication management alone at 5-year median follow-upǁ,1
Watch how the GORE® CARDIOFORM Septal Occluder is implanted.
Reliable and safe delivery
With straightforward procedural steps, the GORE® CARDIOFORM Septal Occluder delivery system allows for repositioning and retrieval¶¶, facilitating reliable and accurate deployment across PFO anatomies.*

The built-in retrieval cord allows for tension-free assessment and retrieval post-lock, if needed.
1 Deploy¶¶
Handle design with slider enables accurate deployment with the ability to reposition.
2 Lock¶¶
Simple-to-use locking mechanism. Occluder is partially released and remains tethered to delivery system.
3 Release¶¶
Pull the retrieval cord until completely removed to release the device from the delivery system.
Life after stroke: Kathryn’s story
Are you young and healthy, but have had a stroke? You are not alone. Kathryn recalls the early, nerve-racking days after her stroke and diagnosis. Learn more about her journey, her decision to close her PFO and life after stroke.
This video was produced for W. L. Gore & Associates by BBC StoryWorks, the commercial content division of BBC Global News.
* All PFO anatomies within indicated sizing parameters of the Instructions for Use.
† 99% effective closure rate across PFO anatomies at 24 months.
‡ Effective closure defined as freedom from large shunt > 25 bubbles as detected by transthoracic echocardiography adjudicated by Echo Core Lab.
§ Data on file 2020; W. L. Gore & Associates, Inc.; Flagstaff, AZ.
II All PFO anatomies were eligible for inclusion into this study within indicated sizing parameters of the Instructions for Use.
¶ Nickel titanium
** Patients allergic to nickel may suffer an allergic reaction to the GORE® CARDIOFORM Septal Occluder device. Certain allergic reactions can be serious; patients should be instructed to notify their physicians immediately if they suspect they are experiencing an allergic reaction such as difficulty in breathing or inflammation of the face or throat. Some patients may also develop an allergy to nickel if this device is implanted. Refer to the Instructions for Use for complete device information, including contraindications, warnings and cautions.
†† As characterized by an in vitro assessment.
‡‡ Reported incidence rate of device-related cardiac erosions for GORE® CARDIOFORM Septal Occluder. Data from CATSWeb Product Surveillance Tracking System (PSTS). June, 2011-February, 2025
¶¶ Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for markets where this product is available. RXOnly
- Kasner SE, Rhodes JF, Andersen G; Gore REDUCE Clinical Study Investigators. Five-year outcomes of PFO closure or antiplatelet therapy for cryptogenic stroke. New England Journal of Medicine 2021;384(10):970-971.
- Lefebvre B, Naidu S, Nathan AS, et al. Impact of echocardiographic parameters on recurrent stroke in the randomized REDUCE PFO cryptogenic stroke trial. Structural Heart 2021;5(4):367-375.
- Søndergaard L, Kasner SE, Rhodes JF, et al.; Gore REDUCE Study Investigators. PFO closure or antiplatelet therapy for cryptogenic stroke. New England Journal of Medicine 2017;377(11):1033-1042.
- Verma DR, Khan MF, Tandar A, et al. Nickel elution properties of contemporary interatrial shunt closure devices. Hours of Invasive Cardiology 2015;27: 99-104.
In some jurisdictions, ASPIRIN is a trademark of Bayer Intellectual Property GmbH or its affiliated companies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® CARDIOFORM Septal Occluder is a permanently implanted device indicated for the percutaneous, transcatheter closure of atrial septal defects (ASDs), such as ostium secundum and patent foramen ovale.
CONTRAINDICATIONS: The GORE® CARDIOFORM Septal Occluder is contraindicated for use in patients:
- Unable to take anti-platelet or anticoagulant medications such as aspirin, heparin, or warfarin.
- With anatomy where the GORE® CARDIOFORM Septal Occluder size or position would interfere with other intracardiac or intravascular structures, such as cardiac valves or pulmonary veins.
- With active endocarditis, or other infections producing bacteremia, or patients with known sepsis within one month of planned implantation, or any other infection that cannot be treated successfully prior to device placement.
- With known intracardiac thrombi.