GORE® PROPATEN® Vascular Graft Clinical Data: Above-knee data
GORE® PROPATEN® Vascular Graft Above-knee bypass primary patency
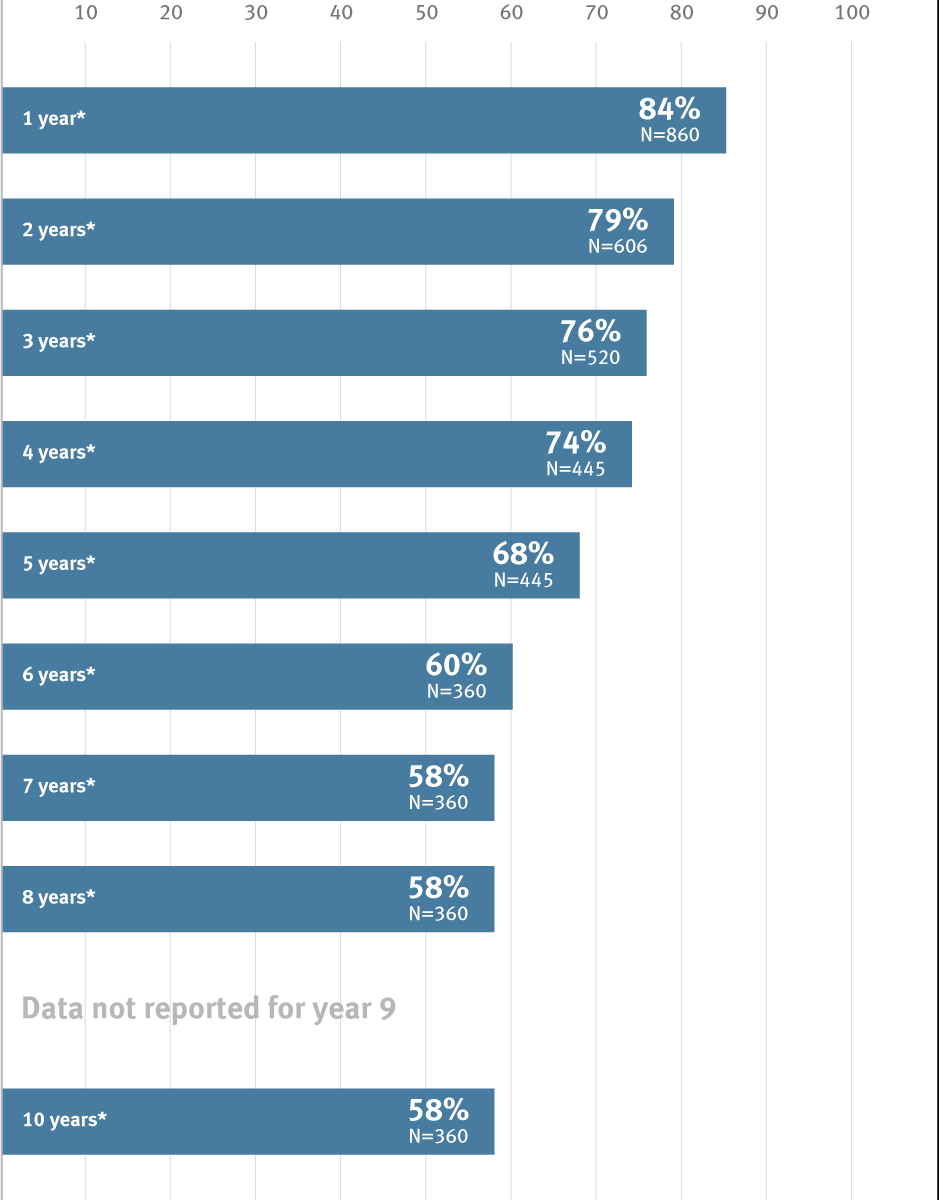
* Overall weighted average primary patency is based on data from 6 peer-reviewed publications meeting pre-determined inclusion criteria.
Study size (N) reflects the initial cohort size of the study.
Above-knee data | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | N | Primary Patency | ||||||||
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 10 | |||
| Piffaretti et al | 2018 | 360 | 82% | 77.50% | 74% | 71% | 64% | 60% | 58% | 58% | 58% |
| Samson et al | 2016 | 87 | 92% | 85% | 85% | 85% | 85% | ||||
| Lindholt et al | 2011 | 112 | 81% | ||||||||
| Daenens et al | 2009 | 86 | 92% | 83% | |||||||
| Peeters et al | 2008 | 75 | 81% | 78% | 75% | ||||||
| Bosiers et al | 2006 | 55 | 84% | ||||||||
Inclusion criteria
Inclusion criteria for above-knee fem-pop GORE® PROPATEN® Vascular Graft data used in this analysis:
- Peer-reviewed clinical journal publications in English language
- Non-overlapping patient populations
- AK bypass primary patency reported for at least 12 months of follow-up
- N = 50 or more AK fem-pop bypass in the GORE® PROPATEN® Vascular Graft treatment arm

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: GORE® PROPATEN® Vascular Graft is intended for use as vascular prostheses for replacement or bypass of diseased vessels in patients suffering occlusive or aneurysmal diseases, in trauma patients requiring vascular replacement, for dialysis access, or for other vascular procedures.
CONTRAINDICATIONS: DO NOT use the GORE® PROPATEN® Vascular Graft in patients with known hypersensitivity to heparin, including those patients who have had a previous incidence of Heparin-Induced Thrombocytopenia (HIT) type II.
DO NOT use any of the below configurations of GORE® PROPATEN® Vascular Graft for coronary artery bypass or cerebral reconstruction procedures:
GORE® PROPATEN® Vascular Graft Integrated Rings
GORE® PROPATEN® Vascular Graft Fixed Ring
GORE® PROPATEN® Vascular Graft Removable Ring
GORE® PROPATEN® Vascular Graft Removable Ring Axillobifemoral
DO NOT use GORE® PROPATEN® Vascular Graft as a patch. If cut and used as a patch, GORE® PROPATEN® Vascular Graft may lack adequate transverse strength.
FOR PATCHING APPLICATIONS: For cardiovascular procedures requiring patch materials, use the appropriate GORE® ACUSEAL Cardiovascular Patch.