GORE® TAG® Thoracic Branch Endoprosthesis
A simplified solution within reach

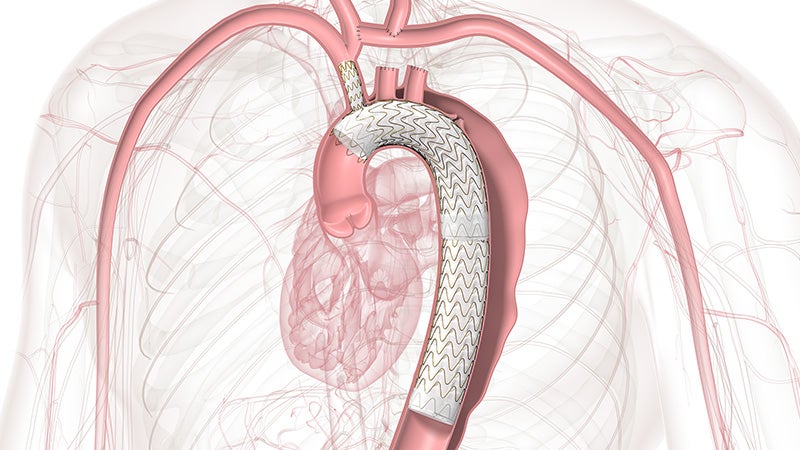
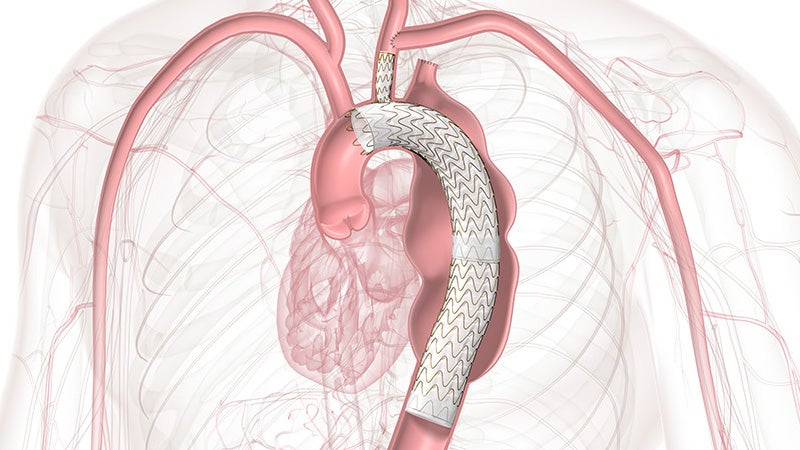
Approved across all aortic arch zones
The off-the-shelf, single branch device physicians trust for Zone 2 branched TEVAR procedures is now also approved for Zones 0 and 1, expanding minimally invasive repair of all lesions involving the aortic arch.
Zones 0 and 1 approved
TBE provides an alternative to open surgical repair and reduces the overall impact of procedures like sternotomy, cardiopulmonary bypass and circulatory arrest.
98.6%
Side branch patency through 1 year1
Zone 0: n = 0 occlusions
Zone 1: n = 1 occlusiona
7.8%
Overall disabling stroke rate through 30 days1
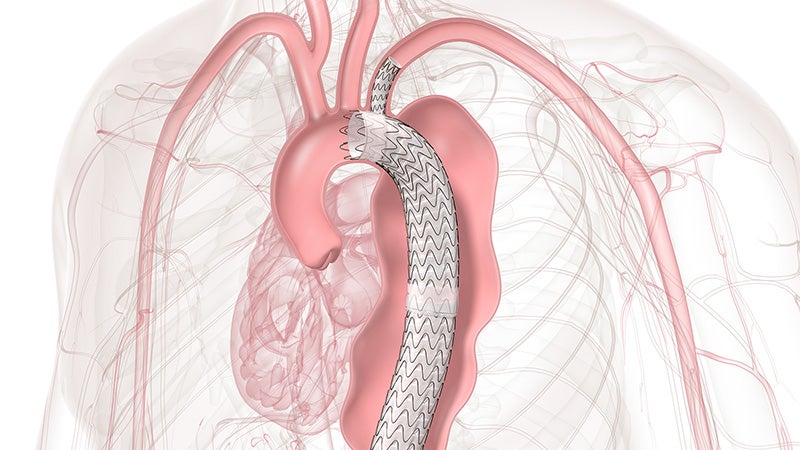
Zone 2 proven
TBE offers a demonstrated solution that preserves flow to the left subclavian artery (LSA) without the potential risks and complexity of surgical revascularization.
99.2%
LSA branch patency through 1 year1
2.9%
Disabling stroke rate, in aneurysm cohort, through 12 months1
Whether it’s Zone 0, 1 or 2, consider TBE for your next aortic arch repair.
How single-branch technology impacted one patient’s life.
Reimbursement & Coding Resources
On-demand, CEU-eligible coding webinars
Explore the latest changes to CPT and MS-DRG codes for thoracic and abdominal aortic endovascular procedures.
- Physician Coding Webinar – Register with ZHealth to access recorded session.
- Hospital Coding Webinar – Register with Libman Education to access recorded session.
More reimbursement & coding resources
Coding is complex. Accuracy is critical. Stay precise with these tools:
- Access the Aortic Arch Repair Reimbursement Guide (PDF) for the latest updates in reimbursement and coding.
- Download our 2026 Hospital Inpatient Coding Guide (PDF) for in-depth coding details
Register now!
January 20, 2026 – ZHealth webinar on new CPT and DRG codes
Contact Us:
Gore Field Reimbursement Directors at + 1-800-248-8489 or fieldreimbursementdirectors@wlgore.com
or
REVENUE CYCLE CODING STRATEGIES® at + 1-888-812-0322
We're here to support you
Related to this product:
a Did not require intervention.
- GORE® TAG® Thoracic Branch Endoprosthesis [Instructions for Use] W. L. Gore & Associates; 2025. MD205141.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE: The GORE® TAG® Thoracic Branch Endoprosthesis is indicated for endovascular repair of lesions of the aortic arch and descending thoracic aorta, while maintaining flow into a single aortic arch branch vessel in patients who have: Adequate iliac/femoral access; Proximal Aortic Landing Zones: For Isolated Lesion Patients: Proximal landing zone cannot be aneurysmal, dissected, heavily calcified or heavily thrombosed; For Dissection Patients: Primary entry tear must be distal to the target branch vessel and the proximal extent of the landing zone must not be dissected; Aortic inner diameter range 16-42 mm; Proximal segment length (length from distal edge of target branch vessel to the midpoint of any proximal branch vessel) of at least 2.0-4.0 cm, depending on Aortic Component selection; Proximal covered length (measured from distal edge of target branch vessel to the distal edge of any proximal branch vessel) of at least 15–36 mm, depending on Aortic Component selection; For patients with prior ascending aorta or aortic arch repair with surgical graft: at least 2 cm landing zone proximal to the distal anastomosis; Target Branch Vessel Landing Zone: Landing zone cannot be aneurysmal, dissected, heavily thrombosed and severely tortuous (180 degree turn within the treated length); Target branch vessel inner diameter of 6–18 mm, depending on Side Branch Portal diameter selected; Target branch vessel minimum length of 2.5–3.0 cm, depending on Side Branch Portal diameter selected. Distal Landing Zone (Isolated Lesion Patients only): Outer curve length must be ≥ 2 cm proximal to celiac artery; Aortic inner diameter range 16-42 mm; Cannot be aneurysmal, dissected, heavily calcified or heavily thrombosed; Native Aorta or previously placed GORE® TAG® Conformable Thoracic Stent Graft.
CONTRAINDICATIONS: The GORE® TAG® Thoracic Branch Endoprosthesis is contraindicated in: Patients with known sensitivities or allergies to the device materials [ePTFE (polytetrafluoroethylene), FEP (fluoroethylpropylene), Nitinol (nickel, titanium), Gold, SB Component only - Heparin (CBAS® Heparin Surface)]; Patients who have a condition that threatens to infect the graft; Patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.