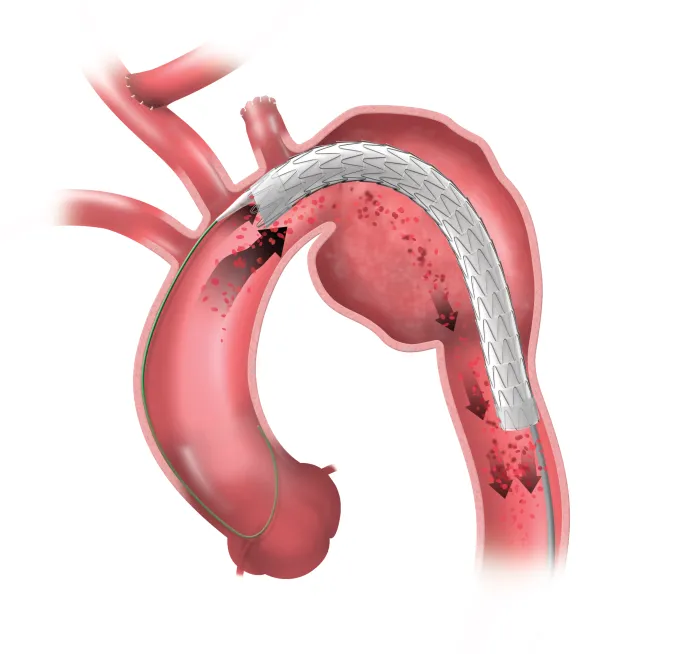
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
A durable, proven stent graft with a delivery system that offers controlled, staged deployment for the endovascular repair of aneurysms, transections and Type B dissections of the thoracic aorta.

The power to be precise
The GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System includes unique angulation control, available at the intermediate stage and then again after full deployment. It is an intuitive system that allows you to focus more fully on your patients.
And now, four new large-diameter tapered devices broaden your treatment options for patients.
Setting a standard of conformability
The Gore device exhibited the lowest spring back in the bench-top analysis.*
*Bench-top evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results.
Related to this product
* Data on file 2023; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- GORE® DRYSEAL Flex Introducer Sheath [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD179444.
- GORE® TAG® Conformable Thoracic Stent Graft [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD164940.
- GORE® TAG® Thoracic Branch Endoprosthesis (TBE) [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. MD184153.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® TAG® Conformable Thoracic Stent Graft is intended for endovascular repair of all lesions of the descending thoracic aorta, including: isolated lesions in patients who have appropriate anatomy, including: adequate iliac/femoral access, aortic inner diameter in the range of 16-42 mm, ≥ 20 mm non-aneurysmal aorta proximal and distal to the lesion; Type B dissections in patients who have appropriate anatomy, including: adequate iliac/femoral access, ≥ 20 mm landing zone proximal to the primary entry tear; proximal extent of the landing zone must not be dissected, diameter at proximal extent of proximal landing zone in the range of 16-42 mm.
CONTRAINDICATIONS: Patients with known sensitivities or allergies to the device materials; patients who have a condition that threatens to infect the graft.