Durable real-world outcomes in AV Access
Treating stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts1
Explore key findings
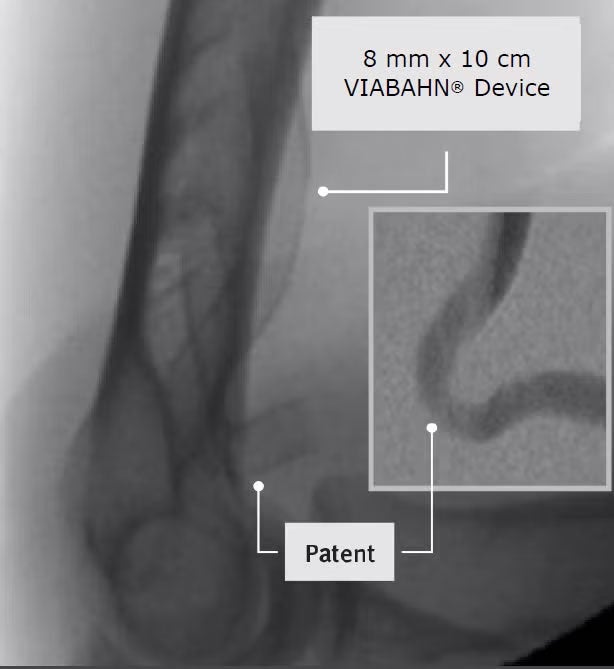
Results from a prospective, real-world multicenter study of the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,† from Japan show high patency at 6 months.
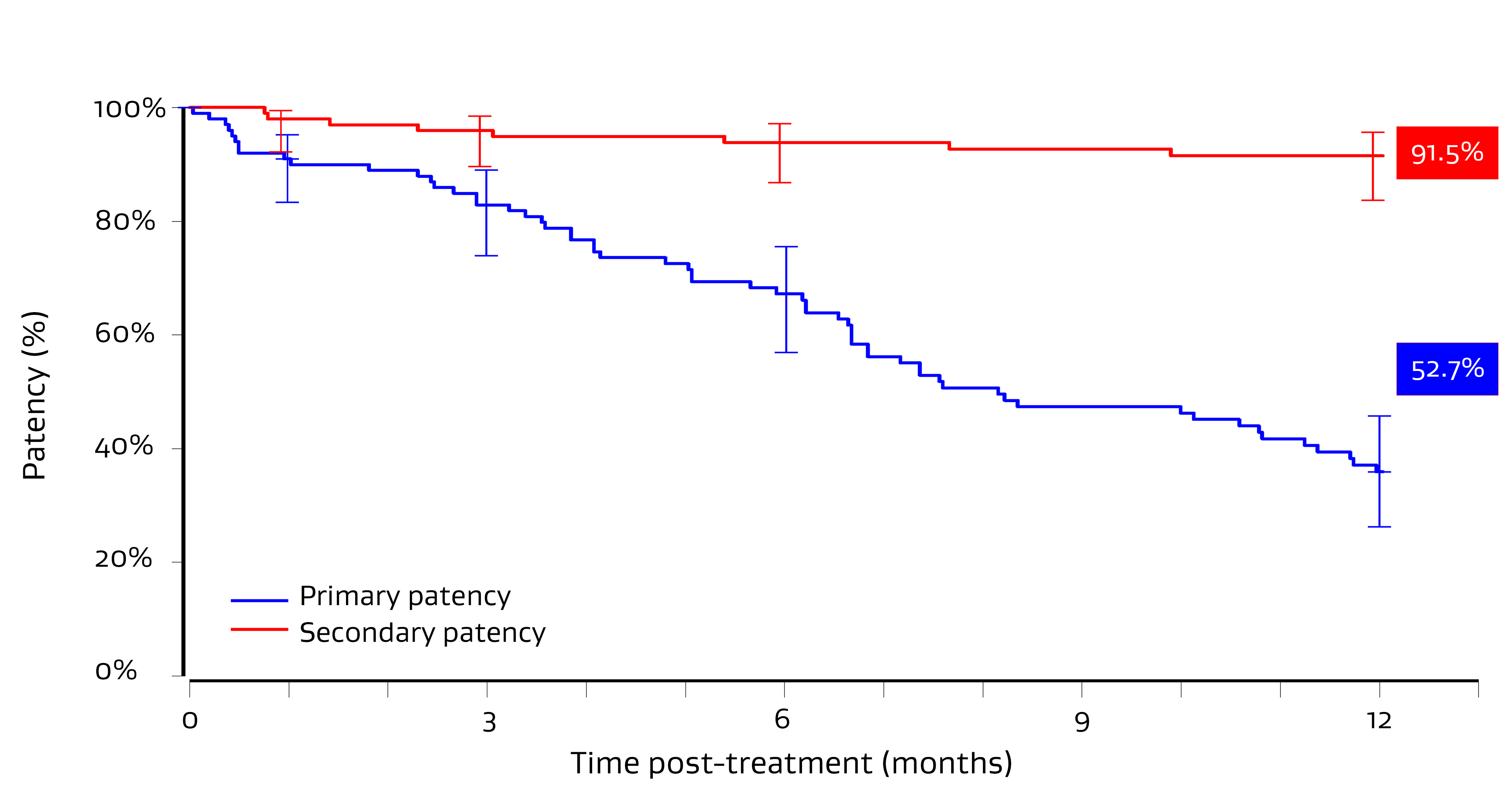
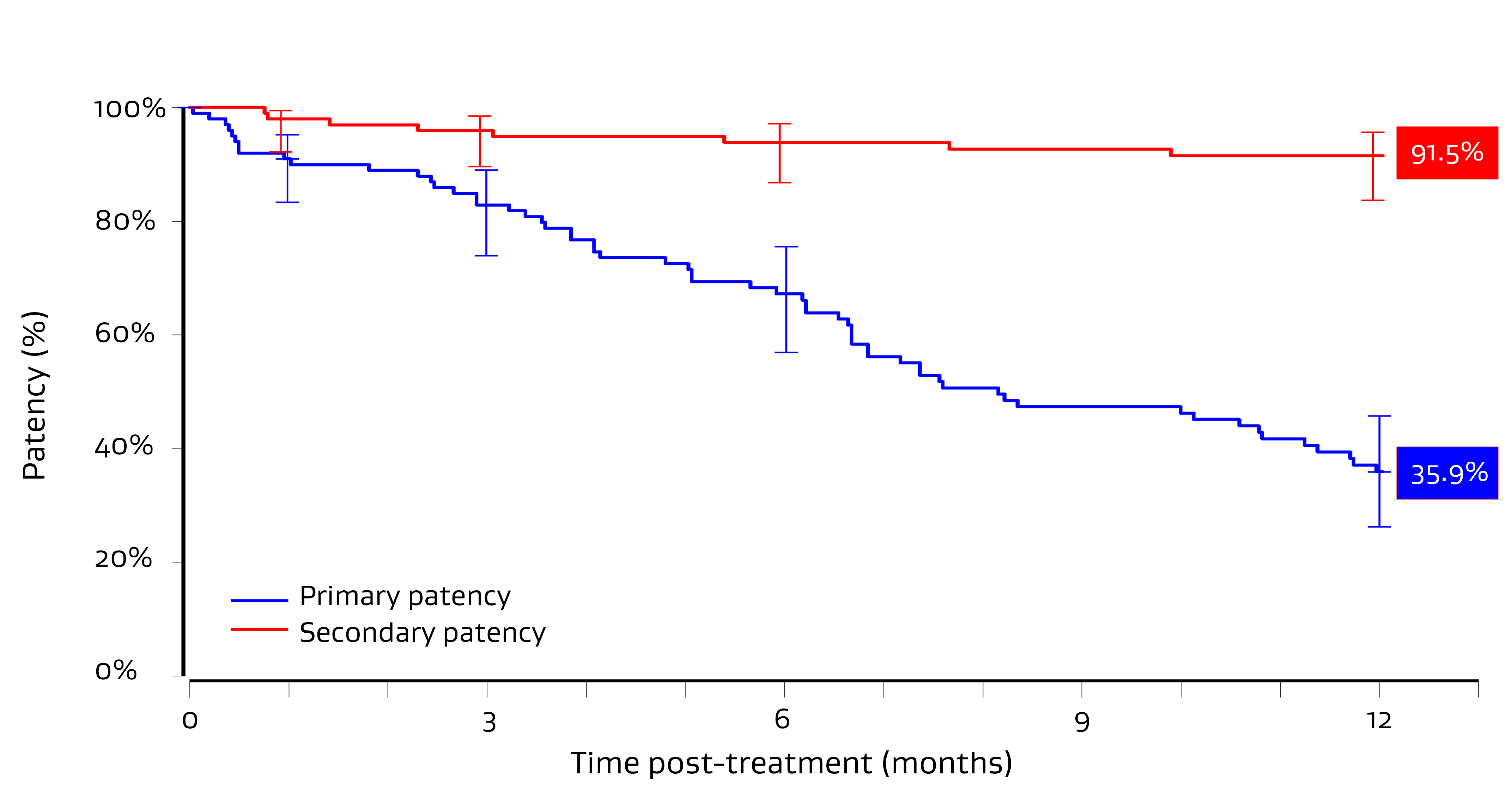
Primary patency1
Secondary patency1
94%
Target lesion
94%
Access circuit
Study patient and lesion characteristics
N = 103 patients
Female: 57%
Average age: 72 years
Average BMI (N = 76): 23 kg/m2
Diabetic: 40%
N = 105 lesions
Average length (N = 93): 43 mm
Percent grafts treated that were thrombosed: 23%
No statistical difference in target lesion primary patency with respect to1:
- Crossing the elbow
- Sex
- Number of prior interventions
- Diabetes
- Age of circuit
- Stent graft size and location
- Stenosis vs. occlusion
- Elephant trunk placement (stent graft in the outflow vein lies without vessel wall apposition)
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
- Haruguchi H, Fukasawa M, Ikeda K, et al. Treatment with a self-expanding endoprosthesis in patients with stenosis or occlusion at the arteriovenous graft: 6-month outcomes of a post-marketing surveillance study. Journal of Vascular Access. In press.
- Fukasawa M, Haruguchi H. A 1-year follow-up analysis of a post market surveillance study of a self-expanding endoprosthesis for stenosis or occlusion at the arteriovenous access. Journal of Vascular Access. In press.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.