GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,†
Proven across a broad range of complex cases, the versatility of the VIABAHN® Device enables you to deliver high patency and durable outcomes to minimize interventions for your patients.
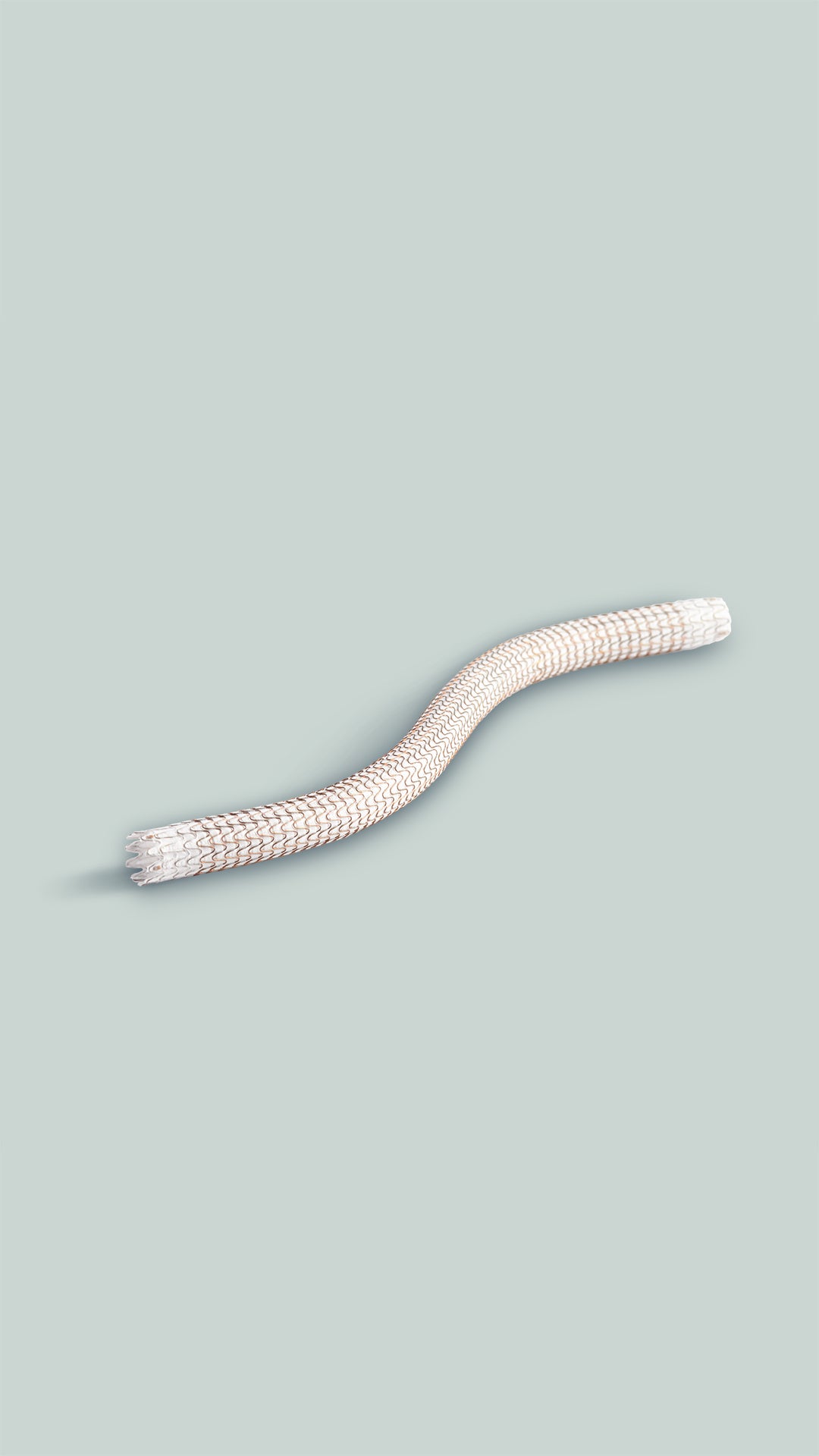
Durable Outcomes. Unmatched Versatility.‡
DURABLE clinical study outcomes in complex cases1
Proven to significantly REDUCE REINTERVENTIONS1
HIGH PATENCY even in the most challenging disease1
A leader among stent grafts with numerous CLINICAL APPLICATIONS for maximum versatility1
RELIABLE PERFORMANCE through decades of partnership with clinicians across the globe1
Backed by a growing body of clinical data, the VIABAHN® Device has become a go-to device for physicians’ most challenging cases.
Over 2,000
publications§
Over 5,650
hospitals using VIABAHN® Device
Over 1,400,000
implanted patients worldwide||
Performance evolution through collaboration
The VIABAHN® Device is a leader among stent grafts. Decades of partnership with clinicians around the globe has resulted in reliable performance across multiple indications. The VIABAHN® Device features:
- Lengths up to 25 cm
- Low profile design
- Multiple FDA-approved indications for use
- Radiopaque markers
- Proven CBAS® Heparin Surface technology3
Related to this product
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
‡ Across indications and configurations of covered stents.
§ This number was the result of a literature search run on August 02, 2023. It was run simultaneously against the following databases: BIOSIS PREVIEWS® Database, EMBASE® Database and MEDLINE® Database.
|| Data on file 2022; W. L. Gore & Associates, Inc.; Flagstaff, AZ.
- GORE® VIABAHN® Endoprosthesis. W. L. Gore & Associates, Inc. Accessed August 2, 2023. https://www.goremedical.com/viabahn/references
- Piazza M, Squizzato F, Dall'Antonia A, et al. Outcomes of self expanding PTFE covered stent versus bare metal stent for chronic iliac artery occlusion in matched cohorts using propensity score modelling. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185.
- CBAS® Heparin Surface. W. L. Gore & Associates, Inc. Accessed August 2, 2023. https://www.goremedical.com/cbas/references

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.





