GORE® VIABAHN® Device family
Disease Overview
Join the next generation of care in the treatment of aortoiliac occlusive disease
Aortoiliac occlusive disease (AIOD) – An increasingly complex condition
The incidence of AIOD is increasing,1 leaving more patients vulnerable to a disease that, untreated, can cause pain, tissue loss and amputation.
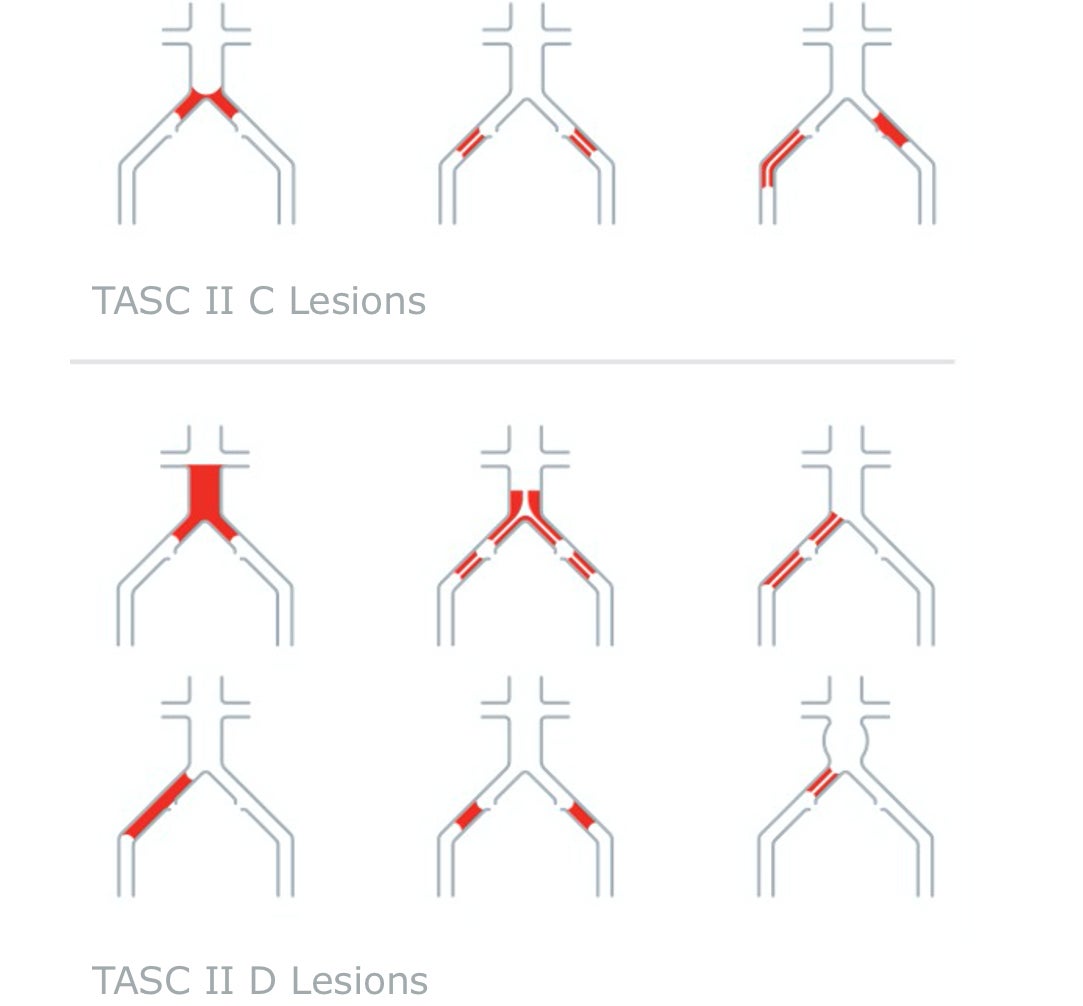
Guidelines for treating AIOD
TASC II A AND B LESIONS:
Guidelines recommend an endovascular approach.2
TASC II C AND D LESIONS:
Guidelines recommend surgery, although multiple consensus and practice guidelines now generally endorse an endovascular-first strategy in experienced endovascular centers.2-5
Potential obstacles of endovascular treatment
Challenges:
Tortuous anatomies
Severe stenosis
Highly calcified occlusions
Risks:
Perforation or rupture of the iliac arteries and aortic bifurcation:
Occurs in nearly 4% of cases
Can be life-threatening6-9
Rupture:
May occur post operatively
Can cause acute bleeding & hypotension10,11
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circulation Research 2015;116(9):1509-1526.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(1)Supplement S:S5-S67.
- Klein AJ, Feldman DN, Aronow HD, et al; Peripheral Vascular Disease Committee for the Society for Cardiovascular Angiography and Interventions. SCAI expert consensus statement for aorto-iliac arterial intervention appropriate use. Catheterization & Cardiovascular Interventions 2014;84(4):520-528.
- Rossi M, Iezzi R. Cardiovascular and Interventional Radiological Society of Europe guidelines on endovascular treatment in aortoiliac arterial disease. Cardiovascular & Interventional Radiology 2014;37(1):13-25.
- European Stroke Organisation, Tendera M, et al; ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). European Heart Journal 2011;32(22):2851-2906.
- Jongkind V, Akkersdijk GJ, Yeung KK, Wisselink W. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. Journal of Vascular Surgery 2010;52(5):1376-1383.
- Indes JE, Pfaff MJ, Farrokhyar F, et al. Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysis. Journal of Endovascular Therapy 2013;20(4):443-455.
- Piazza M, Squizzato F, Dall'Antonia A, et al. Outcomes of self expanding PTFE covered stent versus bare metal stent for chronic iliac artery occlusion in matched cohorts using propensity score modelling. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185.
- Squizzato F, Piazza M, Pulli R, et al; ILIACS Registry Group. Covered versus bare metal kissing stents for reconstruction of the aortic bifurcation in the ILIACS registry. Journal of Vascular Surgery. In press.
- Sobrinho G, Albino JP. Delayed rupture of the external iliac artery after balloon angioplasty and stent placement. Journal of Vascular & Interventional Radiology 2008;19(3):460-462.
- Allaire E, Melliere D, Poussier B, Kobeiter H, Desgranges P, Becquemin JP. Iliac artery rupture during balloon dilatation: what treatment? Annals of Vascular Surgery 2003;17(3):306-314.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXonly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for use as a bridging stent in branched and fenestrated endovascular aortic aneurysm repair with the following†:
- Approved for use branched aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and polypropylene suture with branch diameters of 6/8 mm and lengths of 18/21 mm.
- Approved for use fenestrated aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and fenestrations with polypropylene suture ranging in diameter from 6–8 mm.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
*As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (BXB catalogue numbers).