GORE® VIABAHN® Device family
Lesions
Join the next generation of care in the treatment of aortoiliac occlusive disease
Durable outcomes even in the most complex cases
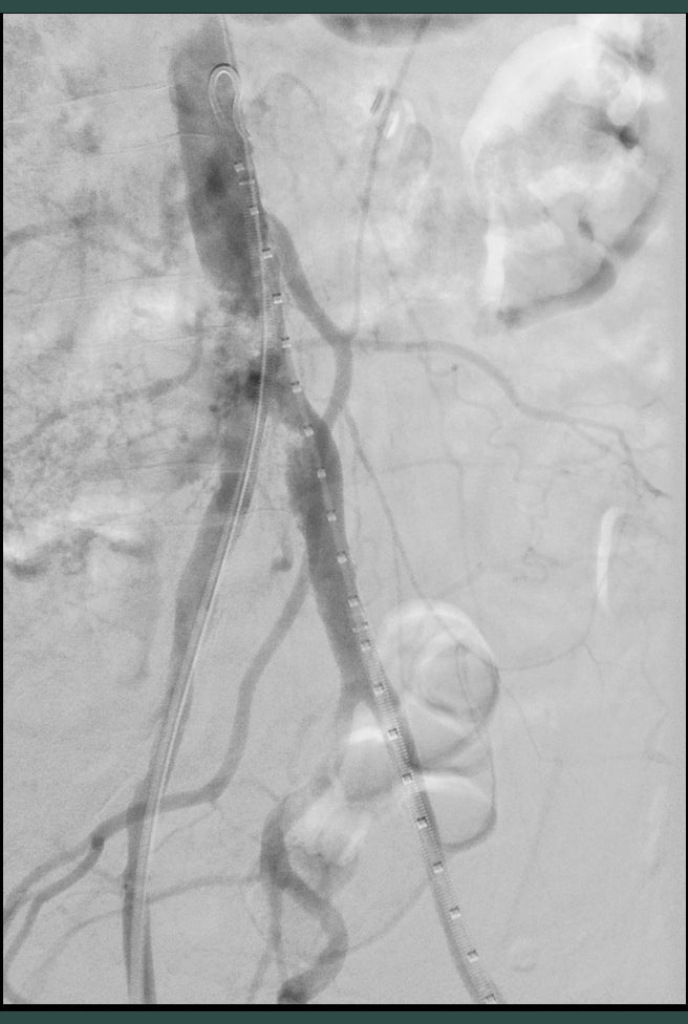
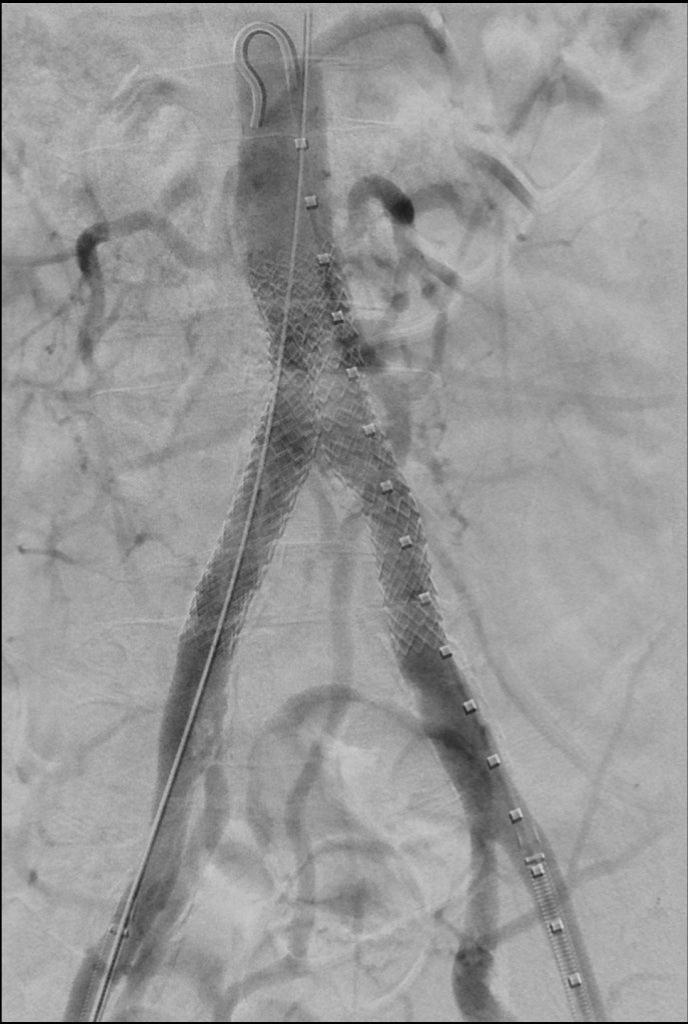
Aortoiliac bifurcation (kissing stent technique) lesions
Common challenges:
- Plaque shift
- Embolization concerns
- Flow dynamics
- Tapered anatomy
- Rupture risk
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) delivers:
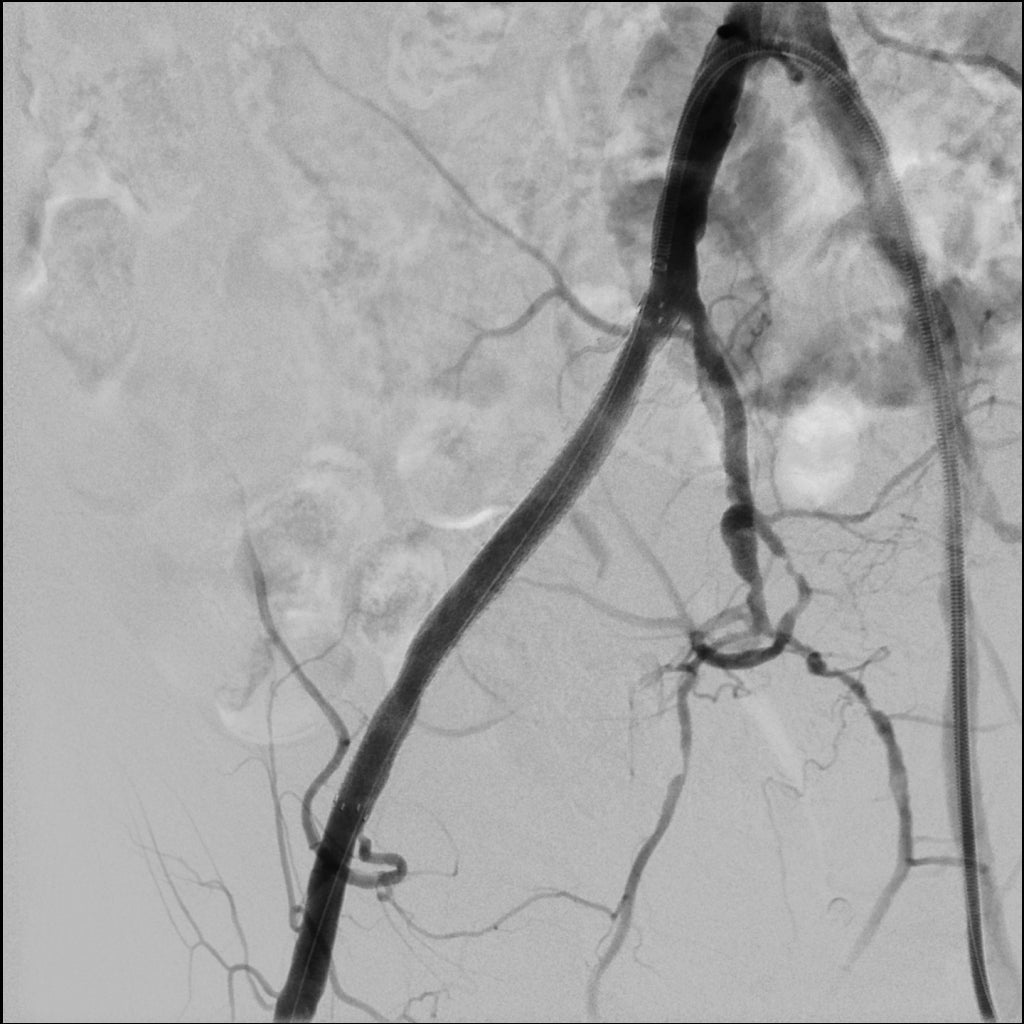
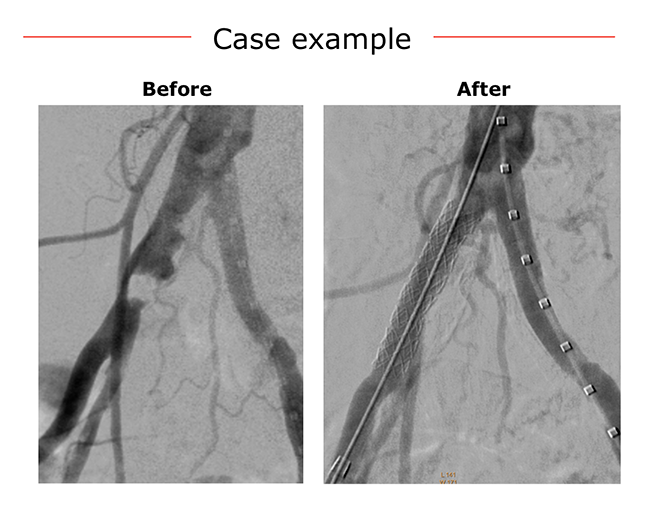
Case example
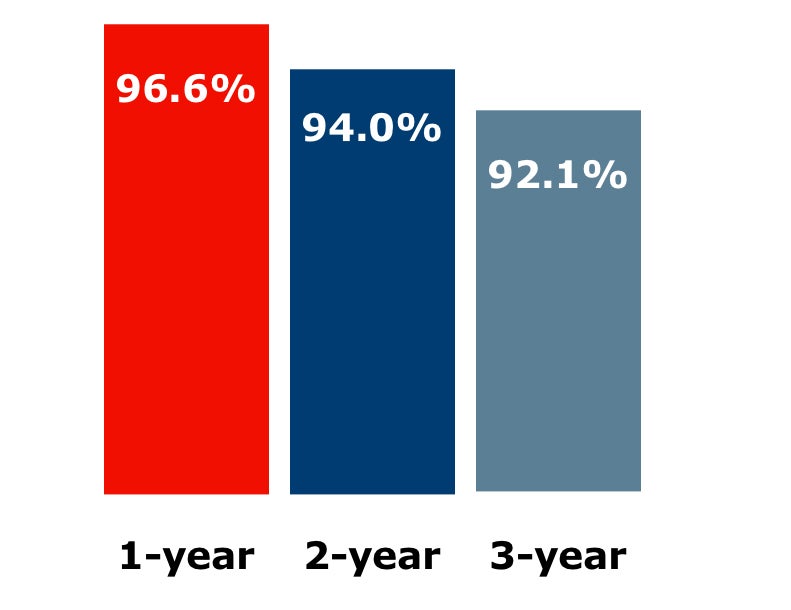
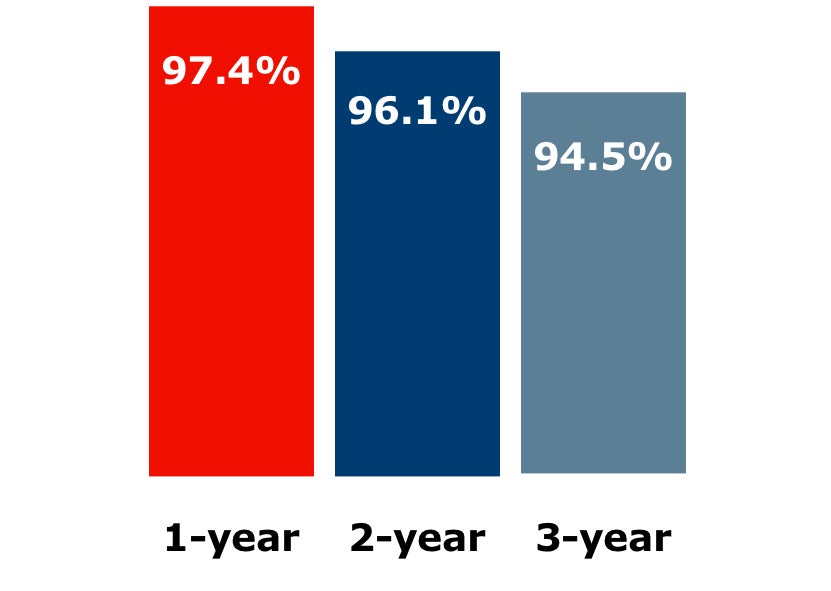
Freedom from target lesion revascularization (fTLR) in kissing stents (N = 118)†
Moderate to severely calcified focal or diffuse lesions
Common challenges:
- Sufficient radial strength
- Embolization concerns
- Need for reintervention
- Rupture risk
The VBX Stent Graft delivers:
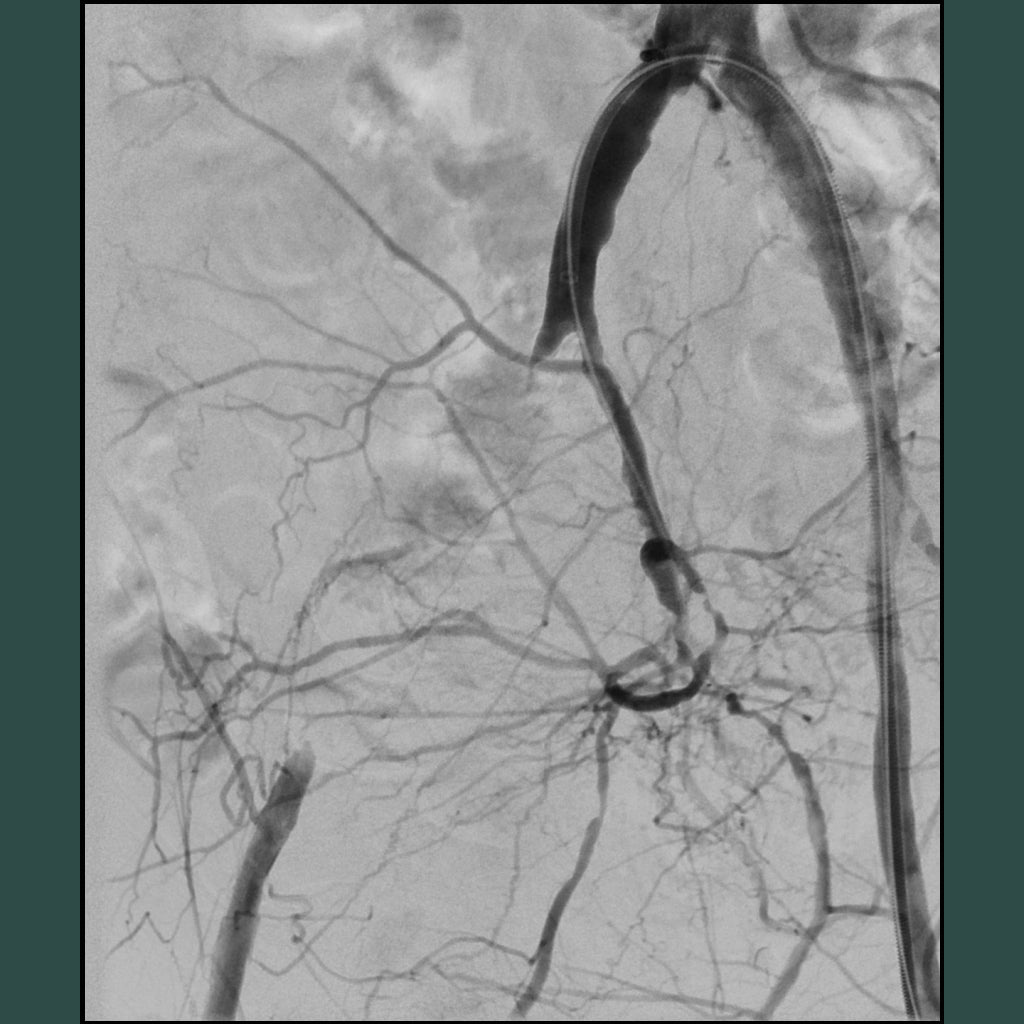
Diffuse iliac artery lesions
Common challenges:
- Embolization concerns
- Conformability
- Need for reintervention (e.g. neointimal hyperplasia)
- Rupture risk
- Deployment accuracy
- Tortuosity
- Vessel movement
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* delivers:
Case example
* As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Data on file 2020; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- CBAS® Heparin Surface. W. L. Gore & Associates website. Accessed February 11, 2021. CBAS references

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXonly
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions found in iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for use as a bridging stent in branched and fenestrated endovascular aortic aneurysm repair with the following*:
- Approved for use branched aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and polypropylene suture with branch diameters of 6/8 mm and lengths of 18/21 mm.
- Approved for use fenestrated aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and fenestrations with polypropylene suture ranging in diameter from 6–8 mm.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
* Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (BXB catalogue numbers).