Vascular medical devices
Innovative solutions from Gore designed to treat complex vascular disease.
Image
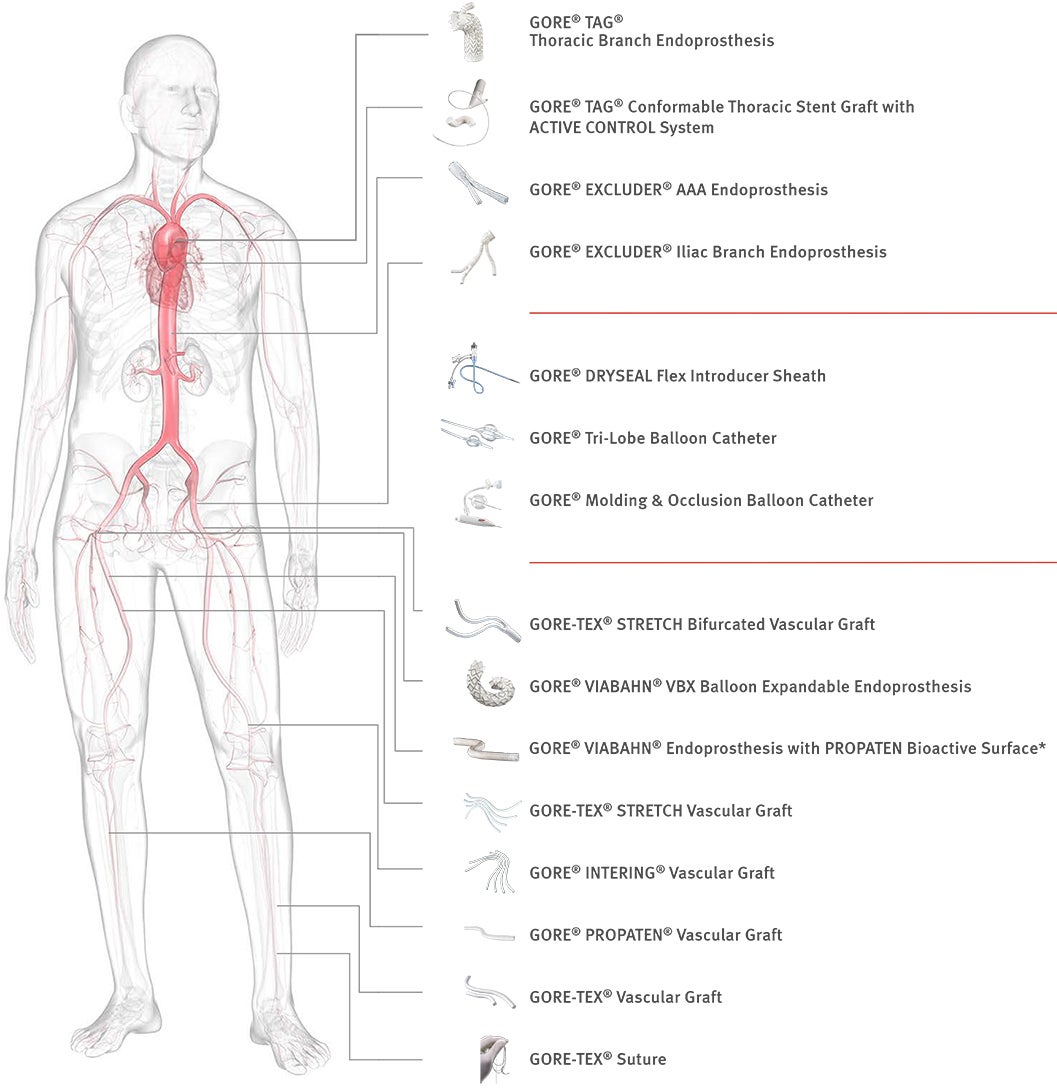
*As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface
Gore information for health care providers that are involved in the purchasing of medical devices

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
Product may not be available in all countries. Please check with your Gore representative for availability.