Innovation for improved outcomes in complex peripheral disease
Gore peripheral arterial disease (PAD) solutions: Innovation for improved outcomes in complex peripheral disease
Our innovative PAD solutions are designed for treating all stages of complex peripheral disease and backed by dedicated service to help improve patient outcomes.
Learn more about our PAD portfolio

GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
Proven procedural success and long-term outcomes through flexibility, strength and accuracy to treat aortoiliac occlusive disease.
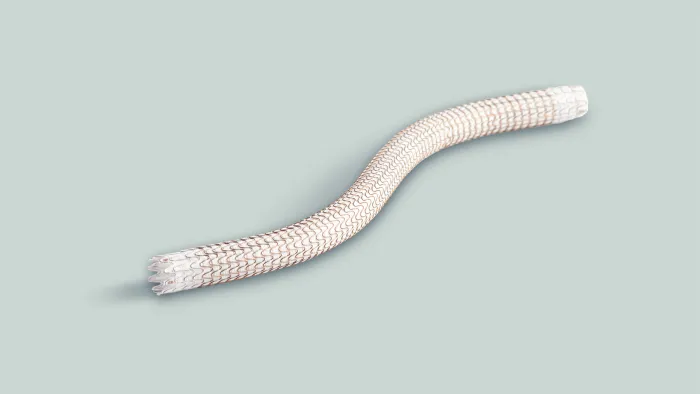
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*
Durable outcomes and unmatched versatility across a broad range of complex cases.
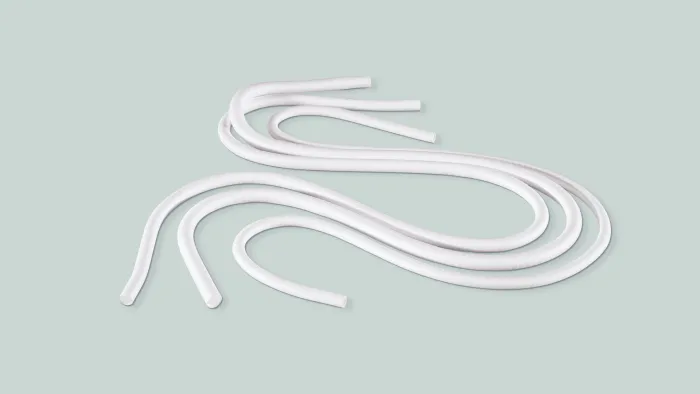
GORE® PROPATEN® Vascular Graft
Proven patency. Measurable value.
Related information
* As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
- Soukas P, Becker M, Stark K, Tepe G. Three-Year Results of the GORE VIABAHN Endoprosthesis in the Superficial Femoral Artery for In-Stent Restenosis. Journal of the Society for Cardiovascular Angiography & Interventions 2023;2(3). https://doi.org/10.1016/j.jscai.2023.100598

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for use as a bridging stent in branched and fenestrated endovascular aortic aneurysm repair with the following:
- Approved for use branched aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and polypropylene suture with branch diameters of 6/8 mm and lengths of 18/21 mm.
- Approved for use fenestrated aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and fenestrations with polypropylene suture ranging in diameter from 6–8 mm.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (Reduced Profile)
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
GORE® PROPATEN® Vascular Graft
INDICATIONS FOR USE IN EUROPE: GORE® PROPATEN® Vascular Graftis indicated for patients who require replacement or bypass of peripheral vessels due to occlusive or aneurysmal diseases or trauma, or for patients who require arteriovenous hemodialysis access in the upper or lower extremities due to end-stage renal disease (ESRD).
GORE® PROPATEN® Vascular Graft Configured for Pediatric Shunt is indicated for staged palliative repair of cyanotic congenital heart disease (CCHD).
CONTRAINDICATIONS: DO NOT use the GORE® PROPATEN® Vascular Graft in patients with known hypersensitivity to heparin, including those patients who have had a previous incidence of Heparin-Induced Thrombocytopenia (HIT) type II.
DO NOT use any of the below configurations of GORE® PROPATEN® Vascular Graft for coronary artery bypass or cerebral reconstruction procedures:
- GORE® PROPATEN® Vascular Graft Integrated Rings
- GORE® PROPATEN® Vascular Graft Fixed Ring
- GORE® PROPATEN® Vascular Graft Removable Ring
- GORE® PROPATEN® Vascular Graft Removable Ring Axillobifemoral
DO NOT use GORE® PROPATEN® Vascular Graft as a patch. If cut and used as a patch, GORE® PROPATEN® Vascular Graft may lack adequate transverse strength.
FOR PATCHING APPLICATIONS: For cardiovascular procedures requiring patch materials, use the appropriate GORE® ACUSEAL Cardiovascular Patch.

