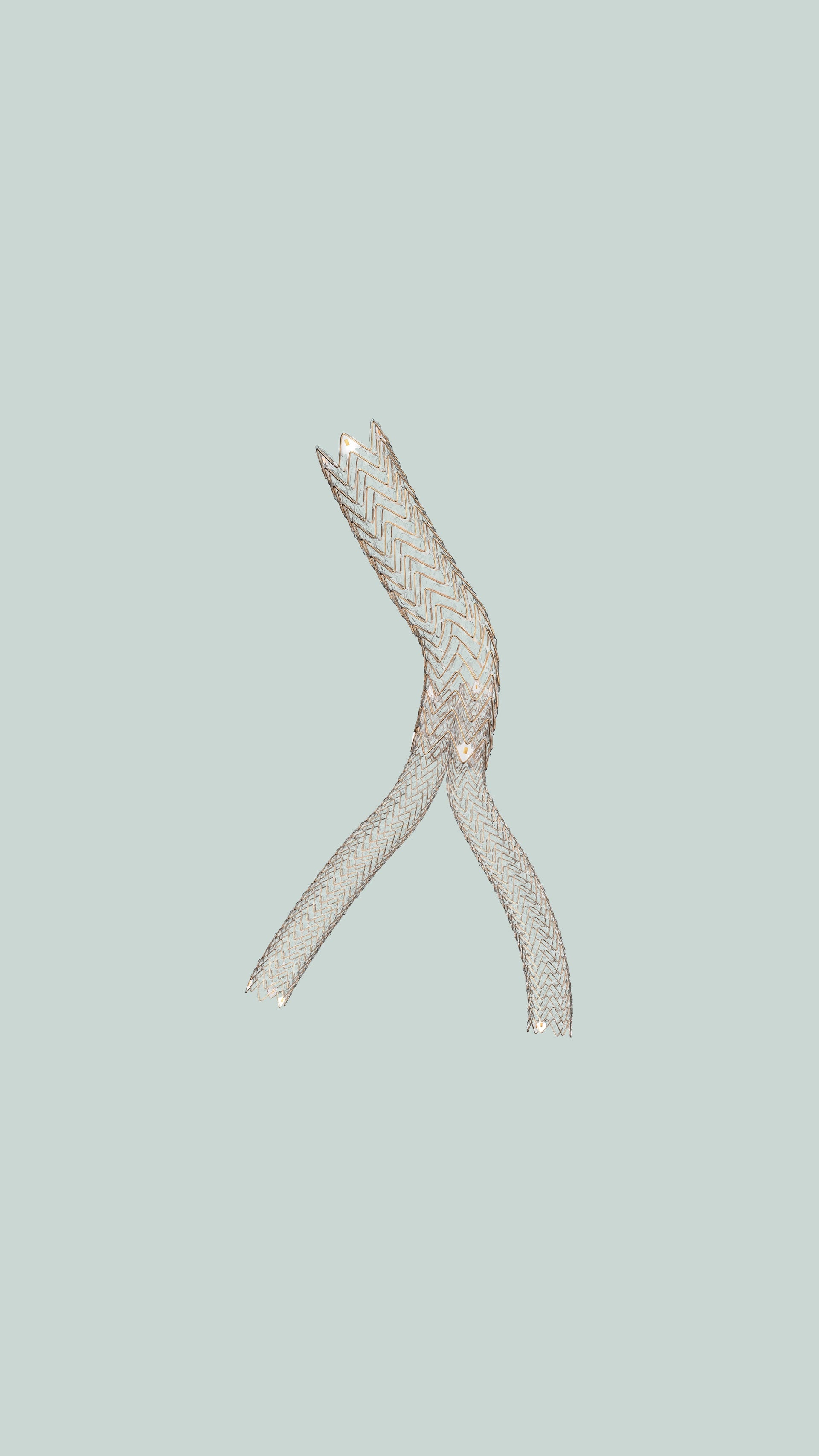
Made to conform, not compress
Experience the first deep venous stent indicated for use in the treatment of inferior vena cava (IVC) obstruction with or without combined iliofemoral obstruction. Purpose-built for iliocaval obstruction, the FORTEGRA Venous Stent combines proven Gore technology and a unique design to deliver an optimal balance of conformability and compression resistance — with the high flexibility you need to treat with confidence in high-movement areas of anatomy.
Made for patients with deep venous disease
Engineered with proprietary Gore materials, with an open-structure, self-expanding nitinol wire frame and expanded polytetrafluoroethylene (ePTFE) polymer lattice, designed for conformability, strength and flexibility.
Proven effective and safe1
In the first venous stent study of its kind to include patients with extensive disease burden—notably all thrombotic patients (acute, subacute and post-thrombotic syndrome)—the FORTEGRA Venous Stent met the primary endpoint composite score for efficacy and safety at 12 months.
Patency results at 12 months
83.4%
primary patency
95.1%
secondary patency
Safety results
0%
embolizations
0%
migrations
0%
fractures
0%
vascular injury or clinically significant pulmonary embolism
0%
device-related deaths or major bleeding through 30 daysa
Made to help more patients
The FORTEGRA Venous Stent offers a broad size range, empowering physicians to treat more vessels and a wider spectrum of deep venous disease.
| Labeled device diameter (mm) | Recommended vessel diameter (mm) | Introducer sheath size (Fr)b | Available device lengths (mm) | Guidewire diameter (Stiff) |
|---|---|---|---|---|
| 10 | 7–9 | 10 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 12 | 9–11 | 11 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 14 | 11–13 | 11 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 16 | 13–15 | 11 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 18 | 15–17 | 12 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 20 | 17–19 | 12 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 24 | 18–22 | 14 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
| 28 | 22–26 | 14 | 50, 75, 100, 150 | 0.035" (0.889 mm) |
Related to this product
a One death and four major bleed events were adjudicated as procedure related by the site and clinical events committee (CEC).
b Refer to the Instructions for Use (IFU) for additional information about device sizing and sheath compatibility.
- GORE® VIABAHN® FORTEGRA Venous Stent [Instructions for Use] W. L. Gore & Associates, Inc; 2025. MD205743. Rev. 2.
Gore received Breakthrough Device designation from the FDA for the FORTEGRA Venous Stent. This program helps expedite the development and FDA review of medical devices that offer more effective treatment for life-threatening or irreversibly debilitating disease and conditions.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® FORTEGRA Venous Stent is indicated for use in the treatment of symptomatic inferior vena cava obstruction with or without combined iliofemoral obstruction.
CONTRAINDICATIONS: The GORE® VIABAHN® FORTEGRA Venous Stent is contraindicated for use in patients with lesions where expansion of an angioplasty balloon catheter to minimum GORE® VIABAHN® FORTEGRA Venous Stent recommended vessel diameters was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
GORE® VIABAHN® FORTEGRA Venous Stent is not authorized for use in Canada.