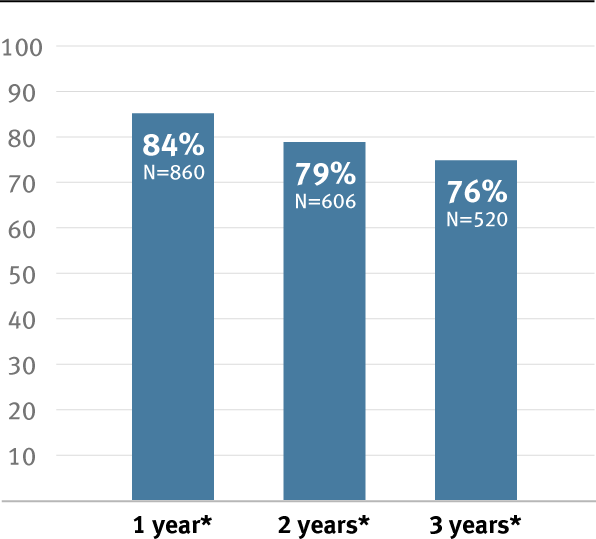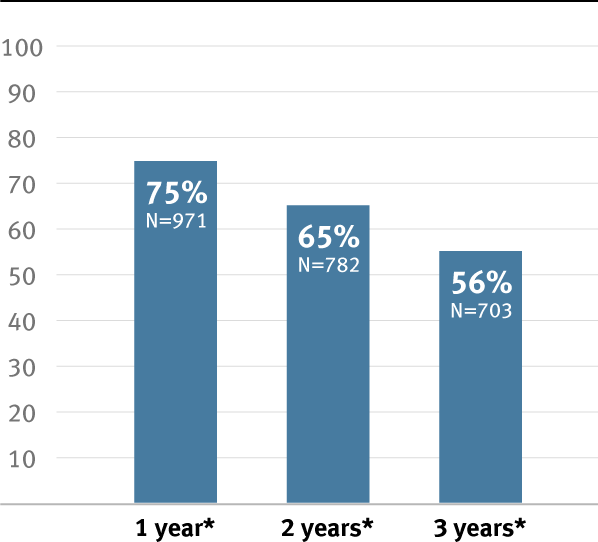Whether facilitating lower extremity revascularization or providing vascular access, the versatile GORE® PROPATEN® Vascular Graft is purposefully designed to improve outcomes and reduce reinterventions. With more than a decade of strong performance, this trusted vascular graft provides proven clinical and economic value.
Bypass procedures: Provide significant benefits in lower extremity bypass procedures compared to standard PTFE grafts.2
Vascular access
Bypass procedures
Leadership in lower extremity bypasses
600K+
Devices implanted
2,000+
Limbs studied
With more than 600,000 devices implanted over a span of more than 10 years ─ and over 2,000 limbs studies ─ the GORE® PROPATEN® Vascular Graft is a clinically proven prosthetic bypass graft solution, both for performance and for low cumulative cost of care.
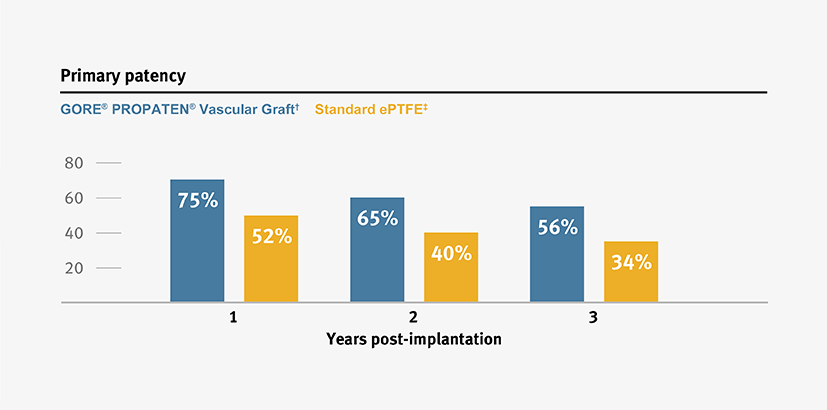
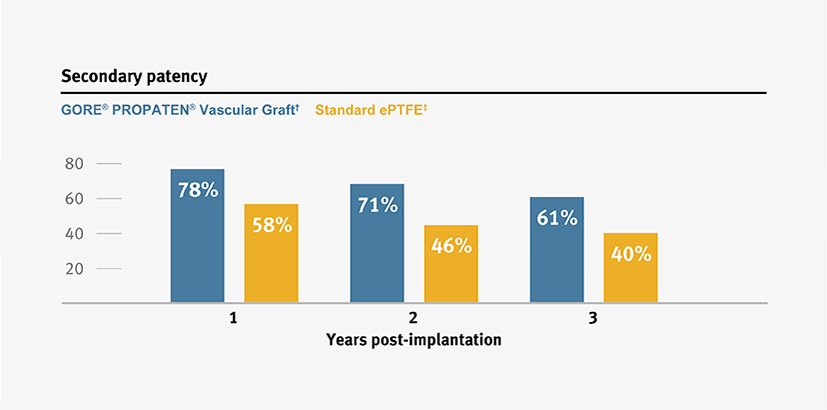
Proven patency
The GORE® PROPATEN® Vascular Graft provides clinical benefits that standard expanded polytetrafluoroethylene (ePTFE) grafts do not.
Fewer occlusions
50% reduction in risk of graft occlusion compared to standard ePTFE in critical limb ischemia (CLI) patients.2
Improved patient outcomes
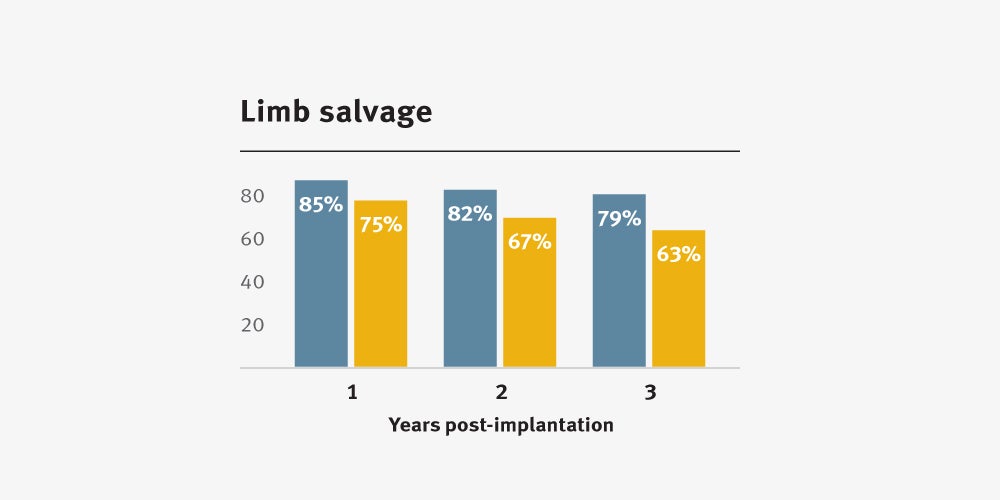
Higher primary and secondary patency, and higher limb salvage for below-knee bypass compared to standard ePTFE from 1–3 years.3
Improved clinical outcomes††
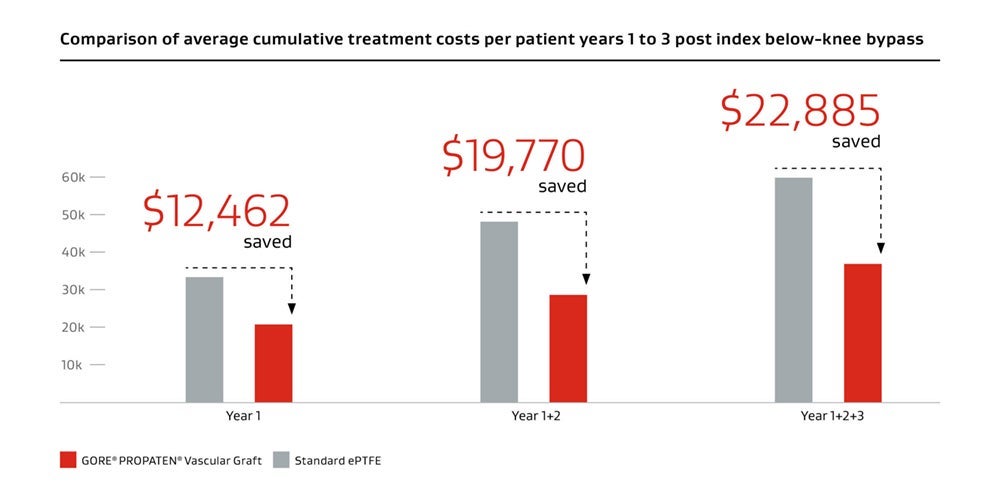
Measurable value
A clinically proven prosthetic bypass graft solution, the GORE® PROPATEN® Vascular Graft also facilitates a low cumulative cost of care.
Lower treatment cost††
Long-term valueII
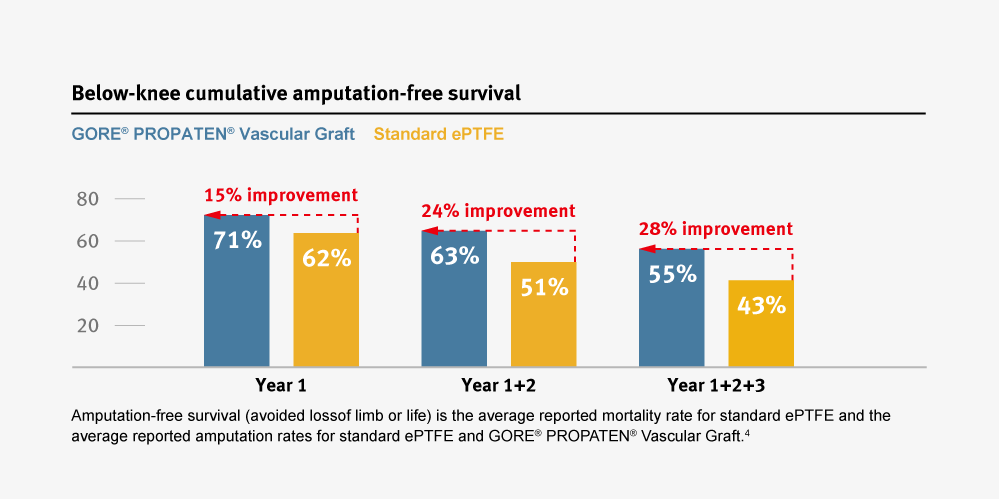
28% Patients
Improvement in amputation-free survival compared to standard ePTFE grafts.
36% Physicians
Decrease in revision procedures compared to standard ePTFE grafts.
38% Providers
Decrease in average costs compared to standard ePTFE grafts.
Vascular access
A trusted choice for vascular access
200K+
Devices implanted
100+
Clinical publications
From fistula-first to patient-first
Todays’ end-stage renal disease (ESRD) patients have greater heterogeneity than ever. That’s one reason 2019 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines5 emphasize the need for more individualized approach to access modality selection.
When your hemodialysis patients are at risk of fistula non-maturation, the GORE® PROPATEN® Vascular Graft can provide confidence and proven results.
- Trusted by physicians more than any other vascular graft6
- Reported potential to improve primary patency by up to 20%**,7
With patency you can count on, you can give your patients reliable vascular access for the road ahead.
- Reduce the need for central venous catheters (CVCs)8
Lasting thromboresistance9
The CBAS® Heparin Surface consists of a proprietary covalent end-point bond that preserves the active site, thus retaining heparin’s anticoagulant activity.
Reported ability to significantly reduce early thrombosis rates**,10
78%
Clot-free survival reported at one year7
Proven Heparin availability
Performance-ready heparin active site.4,11
Proven Heparin bioactivity
Unmatched, persistent ability to take up antithrombin.1,12
Proven Lasting thromboresistance
Improved surface hemocompatibility resulting from heparin availability and bioactivity.1,4,9,11,12
Sustained heparin bioactivity8

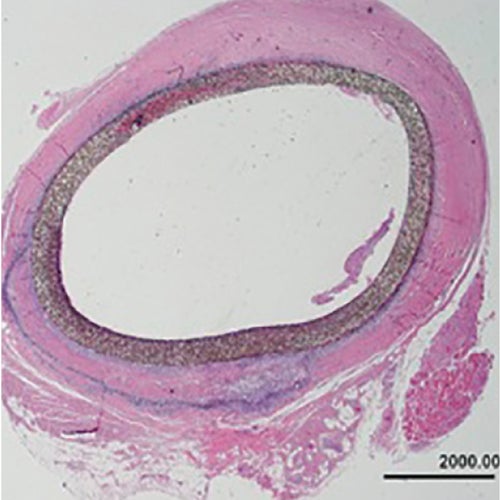
8 years
Explant after 2,939 days
- Femoral to posterior tibial bypass with polyester Linton patch
- Distal anastomosis occluded


4 Years
Explant after 1,553 days (> 4 years)
- Femoropopliteal bypass
Related to this product
* Overall weighted average primary patency is based on data from 14 peer-reviewed publications meeting pre-determined inclusion criteria. Visit goremedical.com/propaten to see inclusion criteria. Visit goremedical.com/propaten to see inclusion criteria, explore the data and see publications.
† Weighted average of GORE® PROPATEN® Vascular Graft data. Visit goremedical.com/propaten to see inclusion criteria, explore the data and see publications.
ǂ Weighted average of standard ePTFE data. Visit goremedical.com/propaten to see inclusion criteria, explore the data and see publications.
§ Procedures for restoring flow in stenosed or occluded graft.
I Based on the three-year published clinical performance and economic model.
¶ Replacing the graft with the new graft.
** Compared with standard non-heparin bonded ePTFE grafts.
†† Data on file, W. L. Gore & Associates, Inc; Flagstaff, AZ.
- Begovac PC, Thomson RC, Fisher JL, Hughson A, Gällhagen A. Improvements in GORE-TEX® Vascular Graft performance by Carmeda® BioActive Surface heparin immobilization. European Journal of Vascular & Endovascular Surgery 2003;25(5):432-437.
- Lindholt JS, Gottschalksen B, Johannesen N, et al. The Scandinavian PROPATEN® Trial – 1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses – a randomized clinical controlled multi-centre trial. European Journal of Vascular & Endovascular Surgery 2011;41(5):668-673.
- GORE® PROPATEN® Vascular Graft. W. L. Gore & Associates Web site.https://www.goremedical.com/propaten/references. Accessed May 15, 2019.
- Gore S, Andersson J, Biran R, Underwood C, Riesenfeld J. Heparin surfaces: impact of immobilization chemistry on hemocompatibility and protein adsorption. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014;102(8):1817-1824.
- Lok CE, Huber TS, Lee T, et al; KDOQI Vascular Access Guideline Work Group. KDOQI Clinical Practice Guideline for Vascular Access: 2019 update. American Journal of Kidney Diseases 2020;75(4)Supplement 2:S1-S164.
- Decision Resources Group (DRG) Market Insights Database. MedTech 360 Dialysis Access Treatment Devices, US; Sept 2020.
- Journal of Vascular Access 2009 Jul-Sept;10(3):153-6. Doi: 10.1177/112972980901000303. PMID: 19670166.
- Al-Balas A, Lee T, Young CJ, Kepes JA, Barker-Finkel J, Allon M. The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. Journal of the American Society of Nephrology 2017;28(12):3679-3687.
- Decision Resources Group (DRG) Market Insights Database. MedTech 360 Dialysis Access Treatment Devices, US; Sept 2020.
- Shenesh D, Goldin I, Hijazi J, et al. A prospective randomized study of heparin-bonded graft (Propaten) versus standard graft in prosthetic arteriovenous access. Journal of Vascular Surgery 2015 Jul;62(1):115-22. Doi:10.1016/j.jvs.2015.01.056. Epub 2015 Mar 12. PMID: 25770987.
- Biran R, Pon D. Heparin coatings for improving blood compatibility of medical devices. Advanced Drug Delivery Reviews 2017;112:12-23.
- Freeman J, Chen A, Weinberg RJ, Okada T, Chen C, Lin PH. Sustained thromboresistant bioactivity with reduced intimal hyperplasia of heparin-bonded PTFE Propaten Graft in a chronic Canine femoral artery bypass model. Annals of Vascular Surgery 2018;49:295-303.
- Biran R. Heparin Activity and Concentration Values from 52 and 96 Month Duration Clinical Explants of the GORE® PROPATEN® Vascular Graft. Flagstaff, AZ: W.L. Gore & Associates, Inc; 2011 [Work plan]. WP104647.
VIZIENT and TECH WATCH are trademarks of Vizient, Inc.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: GORE® PROPATEN® Vascular Graft is intended for use as vascular prostheses for replacement or bypass of diseased vessels in patients suffering occlusive or aneurysmal diseases, in trauma patients requiring vascular replacement, for dialysis access, or for other vascular procedures.
CONTRAINDICATIONS: DO NOT use the GORE® PROPATEN® Vascular Graft in patients with known hypersensitivity to heparin, including those patients who have had a previous incidence of Heparin-Induced Thrombocytopenia (HIT) type II.
DO NOT use any of the below configurations of GORE® PROPATEN® Vascular Graft for coronary artery bypass or cerebral reconstruction procedures:
- GORE® PROPATEN® Vascular Graft Integrated Rings
- GORE® PROPATEN® Vascular Graft Fixed Ring
- GORE® PROPATEN® Vascular Graft Removable Ring
- GORE® PROPATEN® Vascular Graft Removable Ring Axillobifemoral
DO NOT use GORE® PROPATEN® Vascular Graft as a patch. If cut and used as a patch, GORE® PROPATEN® Vascular Graft may lack adequate transverse strength.
FOR PATCHING APPLICATIONS: For cardiovascular procedures requiring patch materials, use the appropriate GORE® ACUSEAL Cardiovascular Patch.