A modern approach to iliac artery stenting
Utilizing the lower profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft)
Case submitted by Peter Faries, M.D. and Brian Leoce, M.D.
New York, New York
Challenge
- 83-year-old male presented post-extensive right lower extremity endovascular revascularization.
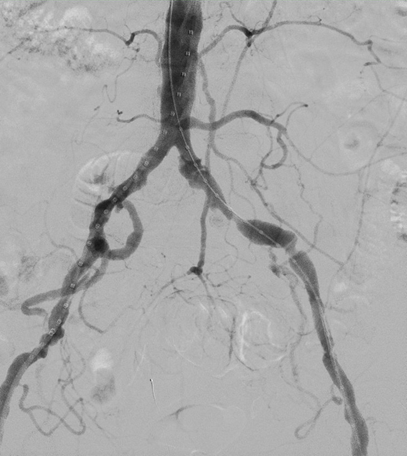
- Aortography demonstrated ectatic left common and external iliac arteries with hemodynamically significant atherosclerotic disease (Figure 1).
- Relevant patient history:
- Diabetes, hypertension, chronic kidney disease, coronary artery disease with prior percutaneous coronary interventions, left transcarotid artery revascularization and peripheral artery disease.
Procedure
- Bilateral common femoral arteries accessed with micropuncture systems.
- Advanced a 10 cm 5 Fr sheath over a wire into right groin followed by 5 Fr x 65 mm pigtail catheter placement in the infrarenal aorta.
- Advanced a 10 cm 6 Fr sheath with a radiopaque tip over wire into left groin followed by placement of a markered diagnostic catheter in the common and external iliac arteries to aid in stent graft sizing.
- Aortography via power injection of the pigtail catheter demonstrated the previously described ectatic left common and external iliac arteries with severe atherosclerotic disease burden as well as a diminutive left hypogastric artery.
- Performed SHOCKWAVE® Intravascular Lithotripsy (IVL) of left common and external iliac arteries using a 5 x 60 mm balloon.
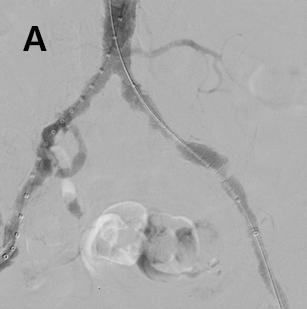
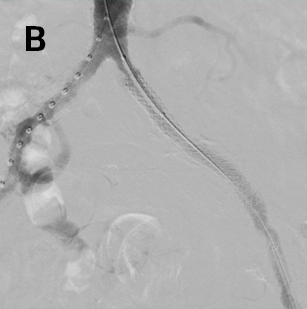
- Advanced a 7 x 79 mm VBX Stent Graft via the 6 Fr left femoral access and successfully deployed in the external iliac artery (Figure 2a), followed by successful deployment of a 7 x 29 mm VBX Stent Graft in the common iliac artery (Figure 2b).
- Serial post-dilation of stent grafts with an 8 x 40 mm and 9 x 20 mm MEDTRONIC® EVERCROSS® PTA Balloon Catheter.
Result
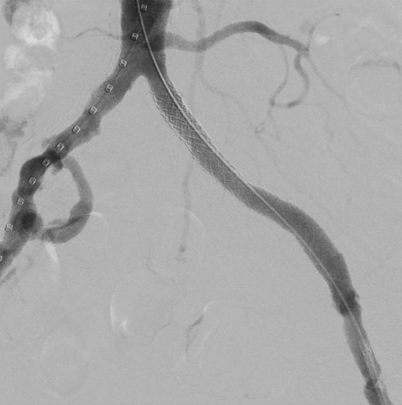
- Technical success was achieved in all aspects of this case and can be seen on completion angiography demonstrating a now widely patent aortoiliac system with good distal flow (Figure 3).
- At 3-week follow-up, patient was neurovascularly intact with no lower extremity or buttock claudication symptoms.
- Bilateral lower extremity duplex ultrasound demonstrated patent in-line flow to the foot bilaterally without abnormal velocities or waveforms.
Case Takeaways
TASC II A lesions are preferentially treated endovascularly.1 Covered stents have demonstrated superior post-intervention ankle-brachial indices (ABI) and reintervention rates versus bare metal stents2, and the VBX Stent Graft has demonstrated excellent technical success as well as long-term patency in the treatment of aortoiliac occlusive disease.3,4
Historically, even the smallest VBX Stent Graft, at 5 mm in diameter, required a 7 Fr profile sheath. However, with a reduction in device profile, the 5, 6 and 7 mm VBX Stent Grafts can now utilize a 6 Fr access sheath (versus legacy device 7 Fr sheath compatibility), while larger stent graft sizes including 8 and 9 mm diameter can now utilize a 7 Fr access sheath (versus legacy device 8 Fr sheath compatibility).
In this case, due to VBX Stent Graft compatibility with a 6 Fr sheath, diagnostic pigtail catheter access was maintained throughout all portions of the case which contributed to successful deployment of the stent graft, obviating the need for hand-held retrograde injections from the treatment side. Utilizing the contralateral side for diagnostic purposes also allows for minimal exchanges through the diseased iliac artery, decreasing the chance of losing wire access or unintentionally disrupting an already heavily diseased vessel.
Images courtesy of Peter Faries, M.D. Used with permission.
- Paisley MJ, Adkar S, Sheehan BM, Stern JR. Aortoiliac occlusive disease. Seminars in Vascular Surgery 2022;35(2):162-171.
- Hajibandeh S, Hajibandeh S, Antoniou SA, Torella F, Antoniou GA. Covered vs uncovered stents for aortoiliac and femoropopliteal arterial disease: a systematic review and meta-analysis. Journal of Endovascular Therapy 2016;23(3):442-452.
- Bismuth J, Gray BH, Holden A, Metzger C, Panneton J; VBX FLEX Study Investigators. Pivotal study of a next-generation balloon-expandable stent-graft for treatment of iliac occlusive disease. Journal of Endovascular Therapy 2017;24(5):629-637.
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.*
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
* Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXB catalogue numbers).
MEDTRONIC and EVERCROSS are trademarks of Medtronic, Inc. SHOCKWAVE is a trademark of Shockwave Medical Inc.