GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
(VBX Stent Graft) Case Studies

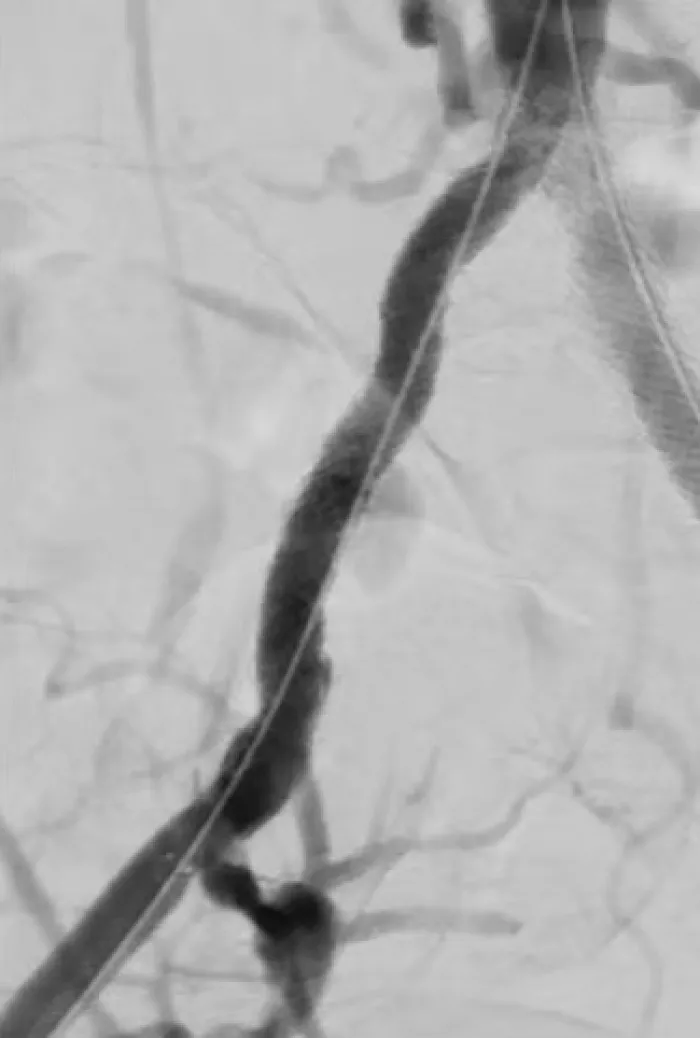
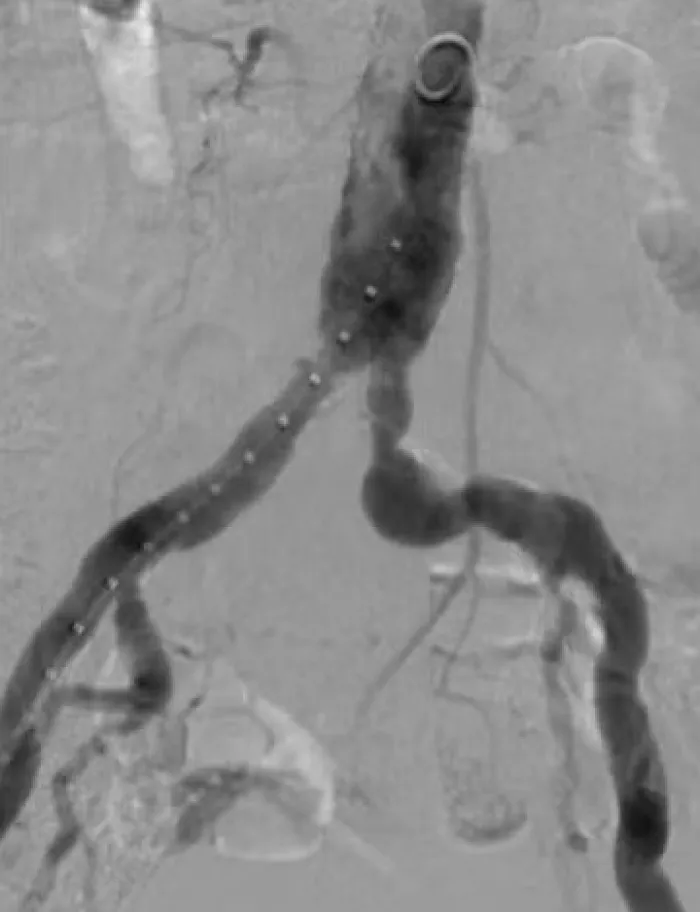
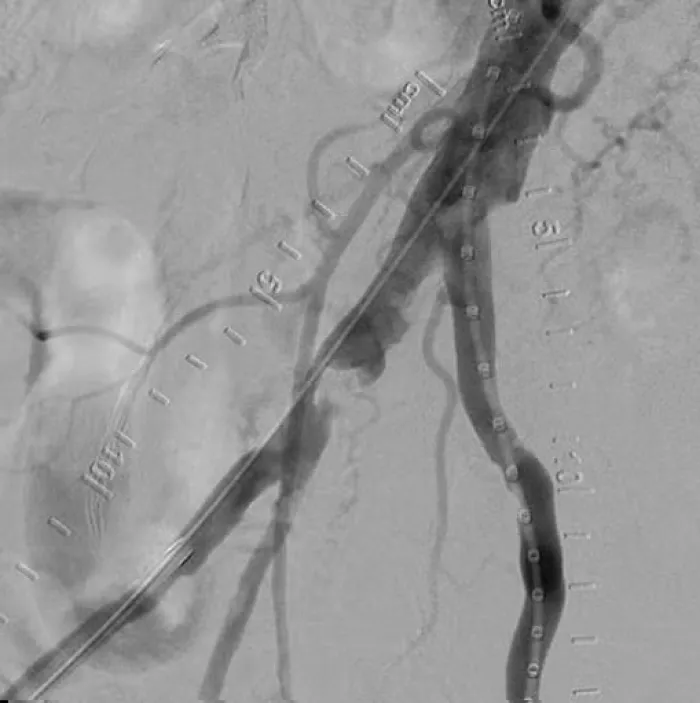
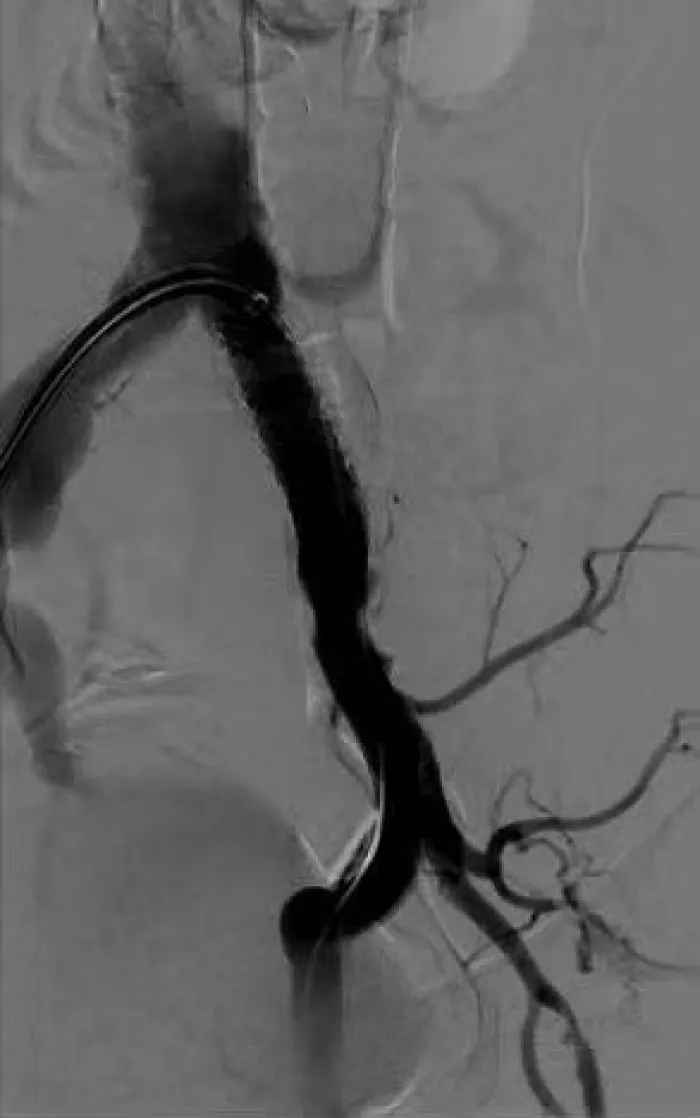
The flexible strength of the VBX Stent Graft helps you address the treatment challenges in complex iliac occlusive disease.
These cases present a range of conditions and procedures demonstrating the proven performance of the VBX Stent Graft. Watch for new cases.
Lower Profile VBX Stent Graft Case Studies
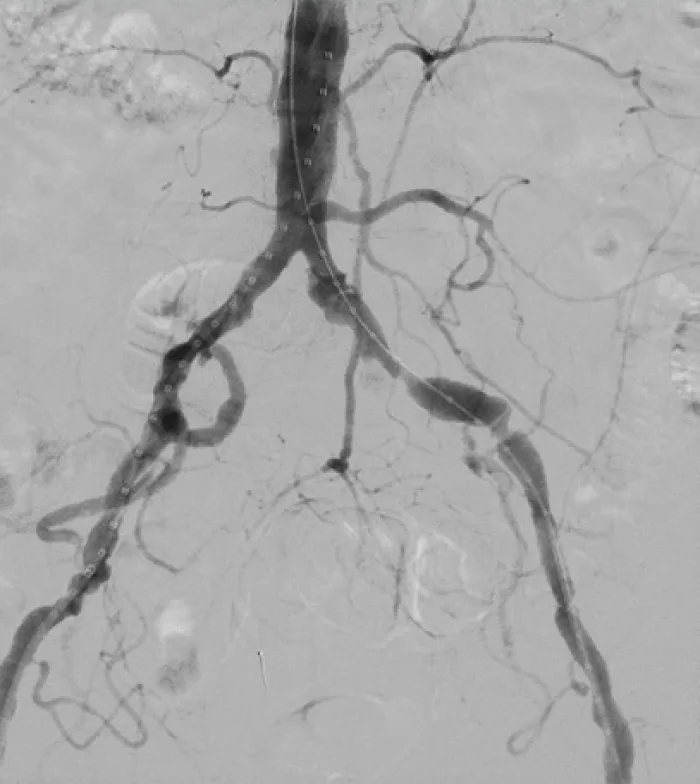
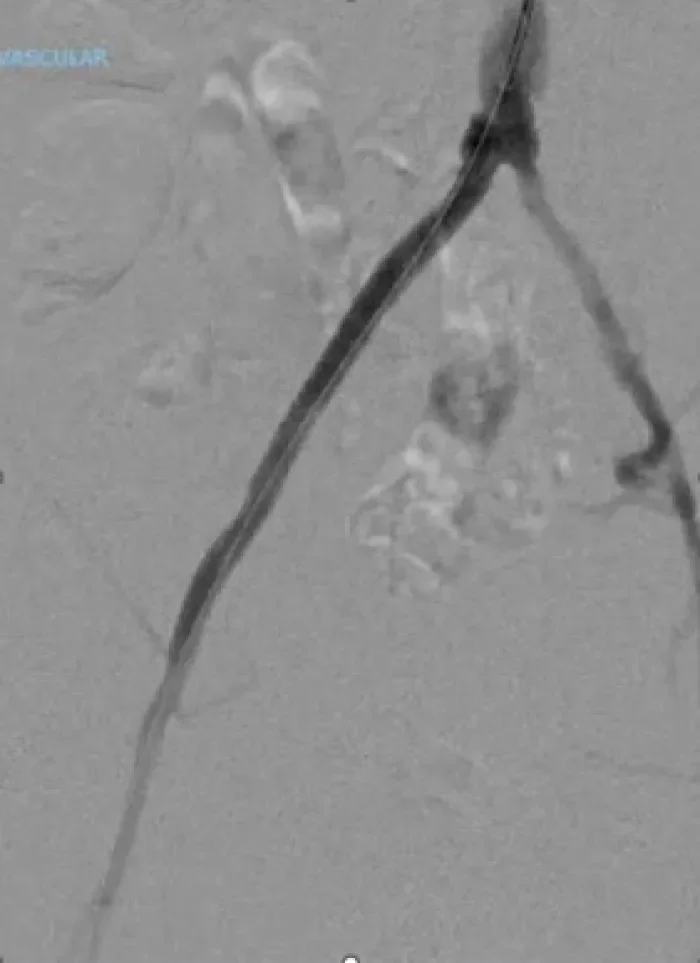
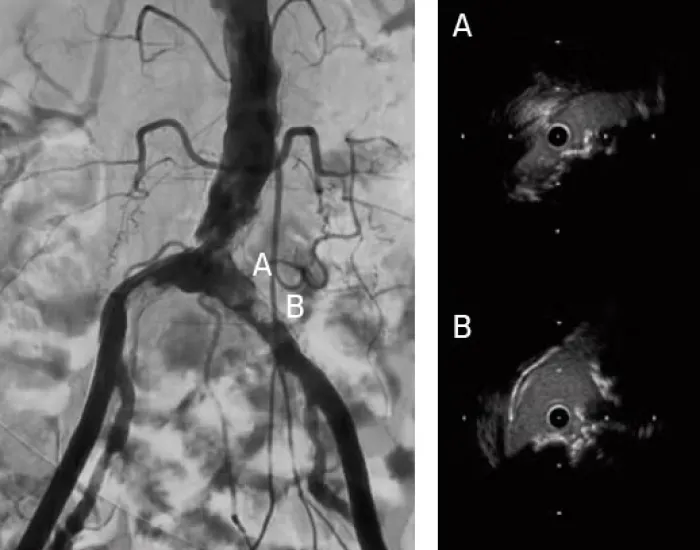
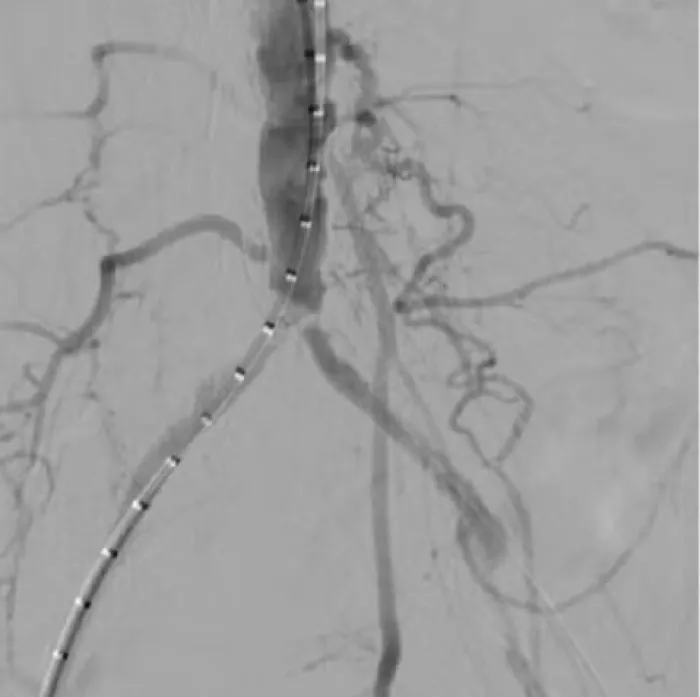
Aortic Bifurcation Lesion Case Studies
Calcified Iliac Lesion Case Studies
Procedural outcomes based on usage of legacy GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXA catalogue numbers).

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is not authorized for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component in Canada.