Radial access approach to iliac artery occlusion
Utilizing the lower profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft)
Case submitted by Edvard Skripochnik, M.D.
New York, New York
Challenge
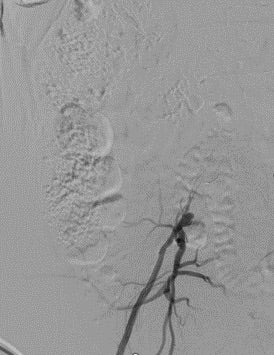
- 76-year-old male presented with a right third digit ulcer and a recurrent left foot wound. Previously treated with left superficial femoral artery (SFA) stenting about 1 year ago. An ultrasound showed occlusion of the left SFA stent and a computed tomography angiogram (CTA) demonstrated right common iliac artery (CIA) occlusion. Angiogram was then attempted via right common femoral artery (CFA) access to revascularize the CIA occlusion and to treat the contralateral SFA occlusion in the same setting. Failed to cross right CIA occlusion. (Figure 1).
- Relevant patient history:
- Former smoker, diabetic, coronary artery disease, prior left SFA revascularization.
Procedure
- Decision was made to attempt a trans-radial approach.
- The left radial artery was evaluated for size and a Barbeau test was performed to confirm safety of this approach.
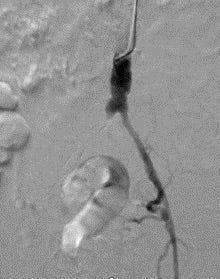
- After ultrasound-guided access of the left radial artery, a 6 Fr x 95 cm TERUMO R2P® DESTINATION SLENDER® Guiding Sheath was positioned just above the aortic bifurcation (Figure 2). Using a .018 TERUMO GLIDEWIRE ADVANTAGE® Guidewire and a TERUMO NAVICROSS® Support Catheter the right CIA occlusion was crossed.
- The lesion was pre-dilated with a .018 4 mm x 80 mm percutaneous transluminal angioplasty (PTA) balloon.
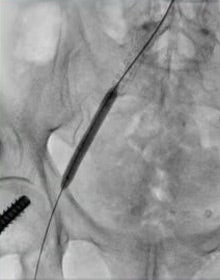
- A 7 mm x 79 mm VBX Stent Graft was deployed across the right CIA and post-dilated to an 8 mm diameter.
- Post-stent angiogram displayed an EIA dissection, likely at the wire re-entry site. An extension 7 mm x 60 mm self-expanding stent was then deployed from the CIA to the EIA and post-dilated with an 8 mm x 80 mm PTA balloon (Figure 3).
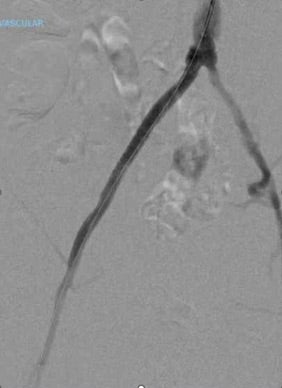
- A completion angiogram demonstrated patent right CIA VBX Stent Graft and EIA stent with no residual stenosis (Figure 4).
Figure 2. From left radial artery approach, a TERUMO R2P® DESTINATION SLENDER® Guiding Sheath is positioned at the aortic bifurcation and the aortogram shows complete occlusion of the right CIA artery. The left iliac system is patent.
Result
- In the same setting, the left SFA occlusion was rechanneled successfully.
- Technical success was achieved in all aspects of this case and can be seen on completion angiography which demonstrates a now widely patent right iliac system with appropriate outflow (Figure 4).
- At 1-month follow-up, the right foot ulcer was healed while the left foot wound was nearly healed, with palpable pedal pulses bilaterally.
Case Takeaways
Iliac artery flush occlusions can pose a significant challenge to revascularization with regard to access site selection, safety and durability. When aggressively managing occlusions and bulky calcified iliac lesions, covered stents provide the necessary safety for potential rupture1, and the VBX Stent Graft has demonstrated excellent long-term patency rates.2,3 It has been demonstrated that the proximal caps of an iliac occlusion can, at times, be more readily crossed.4 Traditionally, when femoral access was unfavorable or when proximal crossing technique was preferred, the options included open brachial artery access, axillary access or open repair. The lower profile VBX Stent Graft, compatible with a 6 Fr sheath on most sizes and still on a 135 cm delivery catheter, allows physicians to not compromise safety and durability when access issues arise. The radial artery approach is now a viable option for treating aortoiliac occlusive disease, as with this case scenario, where treatment was successful, and the contralateral leg could be treated in the same setting.
Images courtesy of Edvard Skripochnik, M.D. Used with permission.
- Society for Vascular Surgery Lower Extremity Guidelines Writing Group, Conte MS, Pomposelli FB, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. Journal of Vascular Surgery 2015;61(3)Supplement:2S-41S.e1.
- Maeda K, Kobayashi T, Emura S, et al. Medium-term outcomes of treatment with a VIABAHN VBX covered stent for aortoiliac occlusive lesions in patients with peripheral artery disease. Annals of Vascular Surgery 2024;105:201-208.
- Holden A, Takele E, Hill A, et al. Long-term follow-up of subjects with iliac occlusive disease treated with the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. In press. https://journals.sagepub.com/doi/10.1177/15266028231165723
- Clair DG, Beach JM. Strategies for managing aortoiliac occlusions: access, treatment and outcomes. Expert Review of Cardiovascular Therapy 2015;13(5):551-563.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.
CONTRAINDICATIONS: Do not use GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.